Sandbox Reserved 653
From Proteopedia
| Line 29: | Line 29: | ||
Kirsch, Jack F. "Mechanism of Action of Aspartate Aminotransferase Proposed on the Basis of Its Spatial Structure." Journal Of Molecular Biology. Science Direct, 15 Apr. 1984. Web. 20 Nov. 2012. <http://www.sciencedirect.com/science/article/pii/0022283684903334>. | Kirsch, Jack F. "Mechanism of Action of Aspartate Aminotransferase Proposed on the Basis of Its Spatial Structure." Journal Of Molecular Biology. Science Direct, 15 Apr. 1984. Web. 20 Nov. 2012. <http://www.sciencedirect.com/science/article/pii/0022283684903334>. | ||
| + | |||
| + | Rose, Bob. "Amino Acid Biosynthesis." Moodle, 15 Nov. 2011. Web. 20 Nov. 2012. <http://moodle.wolfware.ncsu.edu/file.php/30769/Day23_aa_biosynthesis_2012.pdf>. | ||
Thompson, Gregory. "Aspartate Aminotransferase (AST)." Aspartate Aminotransferase. WebMD, 04 Nov. 2011. Web. 20 Nov. 2012. <http://www.webmd.com/digestive-disorders/aspartate-aminotransferase-ast>. | Thompson, Gregory. "Aspartate Aminotransferase (AST)." Aspartate Aminotransferase. WebMD, 04 Nov. 2011. Web. 20 Nov. 2012. <http://www.webmd.com/digestive-disorders/aspartate-aminotransferase-ast>. | ||
Revision as of 02:50, 27 November 2012
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | ||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
Glutamate-Aspartate Aminotransferase
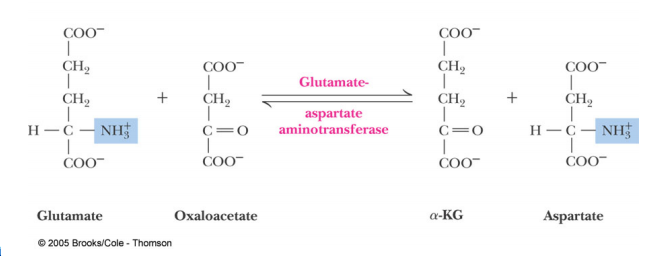
Introduction: Aspartate Aminotransferase(AST)also known as serum glutamic oxaloacetic transaminase (SGOT)is an enzyme that functions within the Amino Acid Biosynthesis pathway to interconvert Glutamate and Aspartate. Aminotransferases work to transform amino acids using transamination reactions. This is a process which utilizes the exchange of an α-keto acid to alter an amino acid usually Glutamate and oxaloacetate to a second amino acid in this case Aspartate and α-ketoglutarate. The reaction is dependent on pyridoxial phosphate (PLP) a cofactor which switches between the Pyridoxial Phosphate(PLP) form and the Pyridoxamine Phosphate (PMP) form. The reaction is important in both amino acid synthesis and degradation. When looking at the formation of aspartate it is synthesized from oxaloacetate which is a key step of the citric acid cycle. Also glutamate can be degraded into ammonium ions through the oxidative deamination process. Aspartate Aminotransferae is generally found and has been studied in E.coli, pig heart cytosol, and chicken mitochondira. It is also present in other microorganisms such as Thermus thermophilus and Haloferax. However within these organisms it is commonly found in the muscles, tissues, heart,liver, and kidneys. This is why Aspartate Aminotransferase is so important in tests that monitor liver disease and damage to any of the above organs.
Structure: The structure of Aspartate Aminotransferase has been determined through studies using X-ray crystallography. AST contains both α-helices packed on either side of a central sheet of β-strands in each of its domains. It is seen that AST has two identical subunits. Each subunit is approximately 300 residues long and each is composed of both a small and large domain. In addition a third domain is present which consists of the N-terminal residues 3-14; these residues link and stabilize the two subunits by forming a strand. Pyridoxial phosphate (PLP), the cofactor for AST binds to the large domain, interacting with the amino group of the Lys258 residue. It also interacts through hydrogen bonding with the Asp222 and Tyr 225 residues within the large domain. The small domain is responsible for the shift of the enzyme from an open conformation to closed conformation. This shift is based on the binding of the substrate. are located between the two domains near the interface. There are two independent active sites each contains two arginine residues which are responsible for the enzyme’s specificity for the substrate. The first is Arg 292 which forms complexes with carboxylate side chains and the second is ARg286 which interacts with the alpha carobxylate group of the substrate.
Implications of Possible Actions: Asparatate Aminotransferase is found in the liver, heart, muscles, kidneys, and other organs. When any of these organs are damaged AST is released and this results in elevated levels of the enzyme in serum. These levels parallel the severity of damage within the organ. Increases in AST levels are common after Heart attack, muscle injury, and Liver disease. In some cases pregnancy can decrease AST levels.Liver damage is commonly detected through a liver function panel which measures the levels of both AST and Alanine Aminotransferase (ALT). When levels of AST exceed the levels of ALT this indicates that the liver disease is most likely alcohol-induced damager, liver tumors, or cirrhosis. When such damage occurs AST levels can take three to six months to return to normal levels.
Kirsch, Jack F. "Mechanism of Action of Aspartate Aminotransferase Proposed on the Basis of Its Spatial Structure." Journal Of Molecular Biology. Science Direct, 15 Apr. 1984. Web. 20 Nov. 2012. <http://www.sciencedirect.com/science/article/pii/0022283684903334>. Rose, Bob. "Amino Acid Biosynthesis." Moodle, 15 Nov. 2011. Web. 20 Nov. 2012. <http://moodle.wolfware.ncsu.edu/file.php/30769/Day23_aa_biosynthesis_2012.pdf>. Thompson, Gregory. "Aspartate Aminotransferase (AST)." Aspartate Aminotransferase. WebMD, 04 Nov. 2011. Web. 20 Nov. 2012. <http://www.webmd.com/digestive-disorders/aspartate-aminotransferase-ast>. |