Malarial Dihydrofolate Reductase as Drug Target
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
---- | ---- | ||
The | The | ||
| - | <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Quadruple_mutant/1'>quadruple mutant</scene> of PfDHFR has four <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Mutated_codons/1'>point mutations</scene>: N51I, C59R, S108N, and I164L. These mutations caused decreased binding affinities of inhibitors similar to pyrimethamine. | + | <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Quadruple_mutant/1'>quadruple mutant</scene> of PfDHFR has four <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Mutated_codons/1'>point mutations</scene>: N51I, C59R, S108N, and I164L. These mutations caused decreased binding affinities of inhibitors similar to pyrimethamine and cycloguanil that have a rigid chlorophenyl substituents. |
</StructureSection> | </StructureSection> | ||
Revision as of 05:51, 28 November 2012
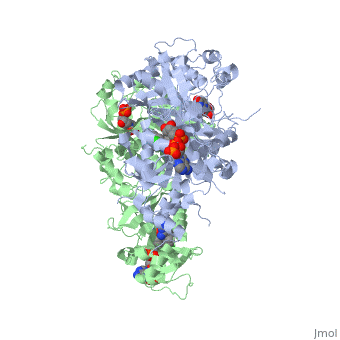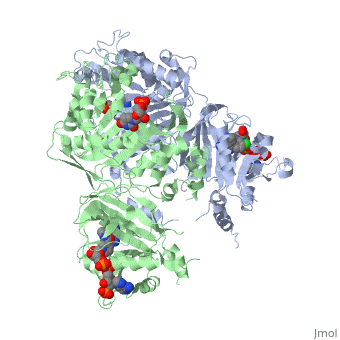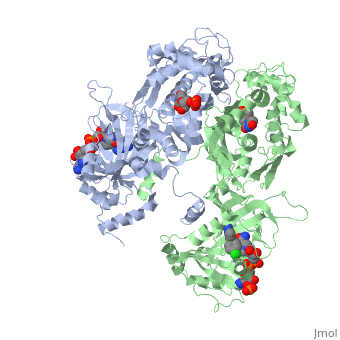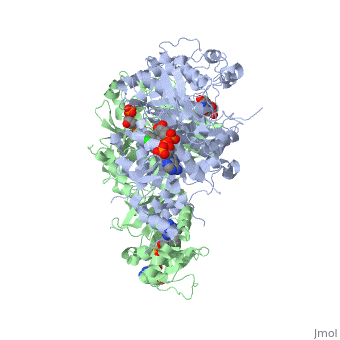
Introduction
There are currently antimalarial drugs that target the malarial dihydrofolate reductase (DHFR) such as pyrimethamine and cycloguanil. However, the effectiveness of these drugs has decreased because of mutations in the enzyme that have led to drug resistance. New research in drug development now incorporates both the wild-type as well as the quadruple mutant DHFR from the Plasmodium falciparum malarial strain.
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Mary Smith, Alexander Berchansky, Karsten Theis, Michal Harel