Malarial Dihydrofolate Reductase as Drug Target
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
---- | ---- | ||
The | The | ||
| - | <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Quadruple_mutant/ | + | <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Quadruple_mutant/2'>quadruple mutant</scene> of PfDHFR has four |
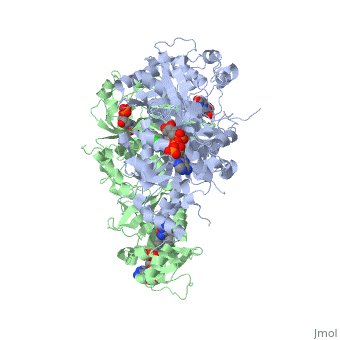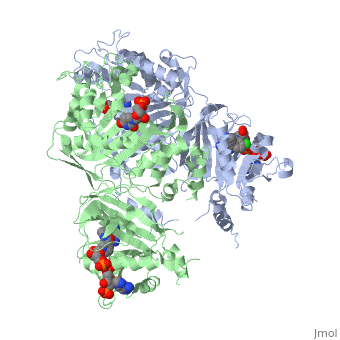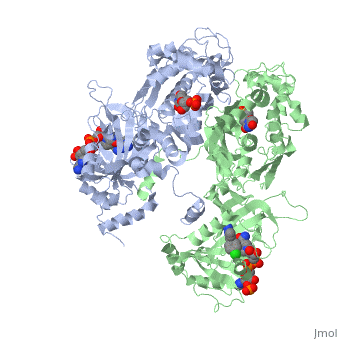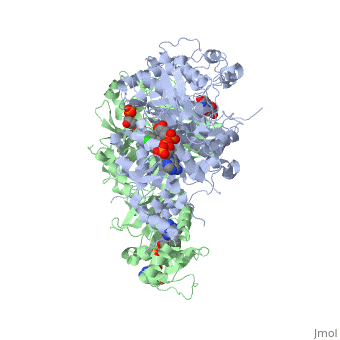
| + | <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Mutated_codons/2'>point mutations</scene>: N51I, C59R, S108N, and I164L<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref>. These mutations cause decreased binding affinities of inhibitors, a huge factor being steric clashing. For pyrimethamine and cycloguanil specifically, the N51I mutation greatly effects their ability to bind the enzyme due to the drugs' rigid chlorophenyl substituents. In an effort to mitigate the effect of the mutations, the commonly occurring quadruple mutant must be addressed in the research of new PfDHFR targeting drugs. | ||
A first step in this direction was to experiment with the drug <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Wr99210/1'>WR99210</scene> that, with its (2,4,5-trichlorophenoxy)propoxy side chain, addressed the steric clash that <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Pyrimethamine_with_wt_pfdhfr/2'>pyrimethamine</scene> was subjected to with a S108N mutation. However further research with this drug was stopped because of its gastrointestinal toxicity in humans. | A first step in this direction was to experiment with the drug <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Wr99210/1'>WR99210</scene> that, with its (2,4,5-trichlorophenoxy)propoxy side chain, addressed the steric clash that <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Pyrimethamine_with_wt_pfdhfr/2'>pyrimethamine</scene> was subjected to with a S108N mutation. However further research with this drug was stopped because of its gastrointestinal toxicity in humans. | ||
Revision as of 08:39, 28 November 2012
Introduction
There are currently antimalarial drugs that target the malarial dihydrofolate reductase (DHFR) such as pyrimethamine and cycloguanil. However, the effectiveness of these drugs has decreased because of mutations in the enzyme that have led to drug resistance. New research in drug development now incorporates both the wild-type as well as the quadruple mutant DHFR from the Plasmodium falciparum malarial strain.
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Mary Smith, Alexander Berchansky, Karsten Theis, Michal Harel