Malarial Dihydrofolate Reductase as Drug Target
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
<scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Mutated_codons/2'>point mutations</scene>: N51I, C59R, S108N, and I164L.<ref>Huang F, Tang L, Yang H, Zhou S, Liu H, Li J, Guo S. Molecular epidemiology of drug resistance markers of Plasmodium falciparum in Yunnan Province, China. Malar J. 2012 Jul 28;11:243. PMID: 22839209</ref> These mutations cause decreased binding affinities of inhibitors, a huge factor being steric clashing. For pyrimethamine and cycloguanil specifically, the N51I mutation greatly effects their ability to bind the enzyme due to the drugs' rigid chlorophenyl substituents. In an effort to mitigate the effect of the mutations, the commonly occurring quadruple mutant must be addressed in the research of new PfDHFR targeting drugs. | <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Mutated_codons/2'>point mutations</scene>: N51I, C59R, S108N, and I164L.<ref>Huang F, Tang L, Yang H, Zhou S, Liu H, Li J, Guo S. Molecular epidemiology of drug resistance markers of Plasmodium falciparum in Yunnan Province, China. Malar J. 2012 Jul 28;11:243. PMID: 22839209</ref> These mutations cause decreased binding affinities of inhibitors, a huge factor being steric clashing. For pyrimethamine and cycloguanil specifically, the N51I mutation greatly effects their ability to bind the enzyme due to the drugs' rigid chlorophenyl substituents. In an effort to mitigate the effect of the mutations, the commonly occurring quadruple mutant must be addressed in the research of new PfDHFR targeting drugs. | ||
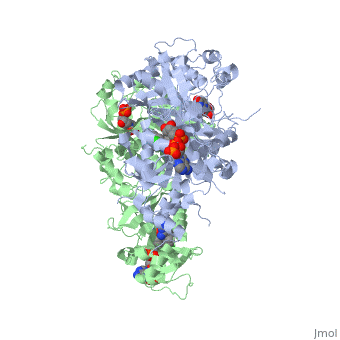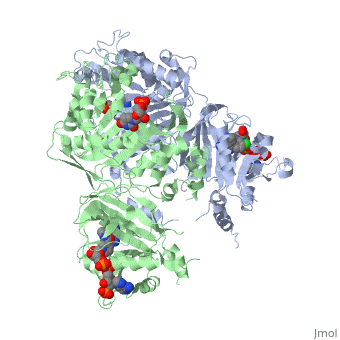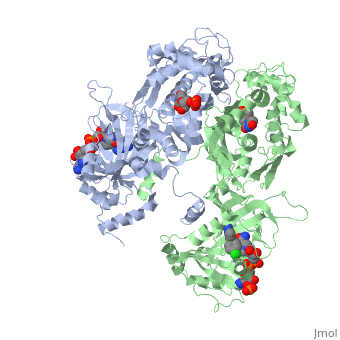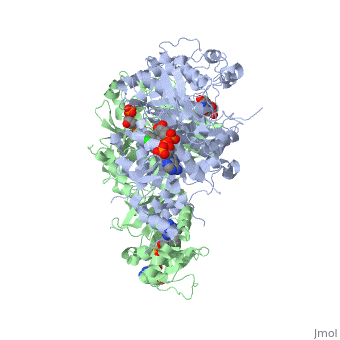
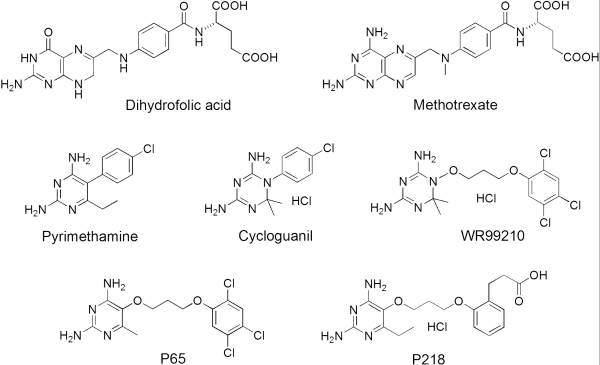
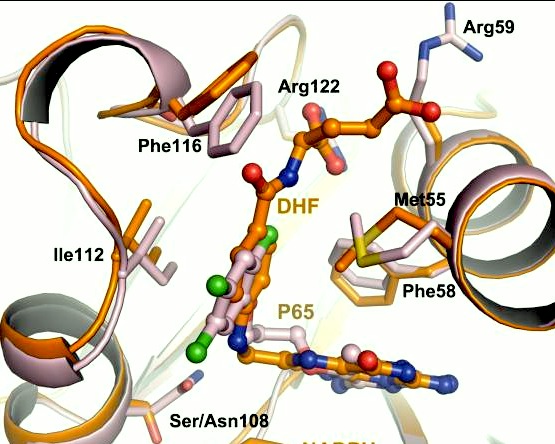
| - | A first step in this direction was to experiment with the drug <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Wr99210/1'>WR99210</scene>, a 4,6-diamino-1,2-dihydro-1,3,5-triazine that, with its (2,4,5-trichlorophenoxy)propoxy side chain, addressed the steric clash that <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Pyrimethamine_with_wt_pfdhfr/2'>pyrimethamine</scene> was subjected to with a Ser108Asn mutation. However further research with this drug was stopped because of its gastrointestinal toxicity in humans and low bioavailbility.<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> Wr99210 was significantly more basic than pyrimethamine, a pyrimidine and slightly acidic substance to match that of the gastrointestinal track, and thus was not readily absorbed in the intestines. When it was compared via in vitro and in vivo experiments to P65, a less basic compound with a 2,4-diaminopyrimidine scaffold, P65 was shown to have significantly higher absorption in vivo. Thus, P65 was chosen as the basis for more research and the later discussed drug, P218. Though Wr99210 was effective at getting deep into the mutant PfDHFR active site, its ineffectiveness as an oral drug would have made it unsuccessful. | ||
| - | + | [[Image:Fig1.jpeg]]<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> | |
| + | A first step in this direction was to experiment with the drug <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Wr99210/1'>WR99210</scene>, a 4,6-diamino-1,2-dihydro-1,3,5-triazine that, with its (2,4,5-trichlorophenoxy)propoxy side chain, addressed the steric clash that <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Pyrimethamine_with_wt_pfdhfr/2'>pyrimethamine</scene> was subjected to with a Ser108Asn mutation. However further research with this drug was stopped because of its gastrointestinal toxicity in humans and low bioavailbility.<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> Wr99210 was significantly more basic than pyrimethamine, a pyrimidine and slightly acidic substance to match that of the gastrointestinal track, and thus was not readily absorbed in the intestines. When it was compared via in vitro and in vivo experiments to P65, a less basic compound with a 2,4-diaminopyrimidine scaffold, P65 was shown to have significantly higher absorption in vivo. Thus, P65 was chosen as the basis for more research and the later discussed drug, P218. Though Wr99210 was effective at getting deep into the mutant PfDHFR active site, its ineffectiveness as an oral drug would have made it unsuccessful. | ||
| - | [[Image:Fig1.jpeg]]<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> | ||
| + | [[Image:Fig22.jpeg]]<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> | ||
| - | </StructureSection> | ||
| - | P218 | + | |
| + | P218 | ||
---- | ---- | ||
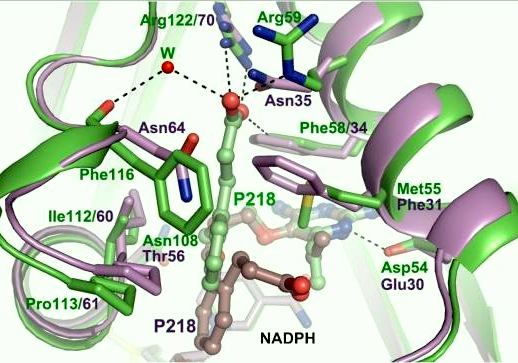
| - | + | It was resolved that a structure containing 2,4-diaminopyrimidine anchor on a new drug would provide a solution for the steric hindrance present with pyrimethamine because of its rigid chlorophenyl group. In addition to this anchor allowing deep binding into the active site of PfDHFR, the other end of the molecule connected by a flexible linker region, a carboxylate group, would form strong hydrogen bonds with the conserved <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Pfdhfr_arg122/1'>Arg122</scene> residue. These ideas culminated into the formation of P218. | |
| + | There are three regions of the DHFR active site that differ between <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Human_dhfr_and_folate/1'>human</scene> and ''Plasmodium falciparum'' that allow a drug to target the PfDHFR specifically while not harming that of humans. One important such difference is the arginine at position 122 in PfDHFR and 70 in humans. The importance in devising a compound, such as P218, that would interact with that conserved Arg122 residue mentioned above is that the compound would interact with the PfDHFR Arg122 residue while leaving the <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Hdhfr_arg70/1'>human Arg70</scene> and thus human DHFR alone. This design and intent for P218 was shown to be successful. | ||
| + | |||
[[Image:Fig3.jpeg]]<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> | [[Image:Fig3.jpeg]]<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | </StructureSection> | ||
| + | |||
Revision as of 09:43, 29 November 2012
Introduction
There are currently antimalarial drugs that target the malarial dihydrofolate reductase (DHFR) such as pyrimethamine and cycloguanil. However, the effectiveness of these drugs has decreased because of mutations in the enzyme that have led to drug resistance. Since these mutations are becoming much more prevalent in malaria cases, new research in drug development must now incorporates both the wild-type as well as the quadruple mutant DHFR from the Plasmodium falciparum malarial strain, the most common and lethal of the malaria species.[1]
| |||||||||||
References
- ↑ Somsak V, Uthaipibull C, Prommana P, Srichairatanakool S, Yuthavong Y, Kamchonwongpaisan S. Transgenic Plasmodium parasites stably expressing Plasmodium vivax dihydrofolate reductase-thymidylate synthase as in vitro and in vivo models for antifolate screening. Malar J. 2011 Oct 7;10:291. PMID: 21981896
- ↑ Huang F, Tang L, Yang H, Zhou S, Liu H, Li J, Guo S. Molecular epidemiology of drug resistance markers of Plasmodium falciparum in Yunnan Province, China. Malar J. 2012 Jul 28;11:243. PMID: 22839209
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
Proteopedia Page Contributors and Editors (what is this?)
Mary Smith, Alexander Berchansky, Karsten Theis, Michal Harel