Malarial Dihydrofolate Reductase as Drug Target
From Proteopedia
| Line 13: | Line 13: | ||
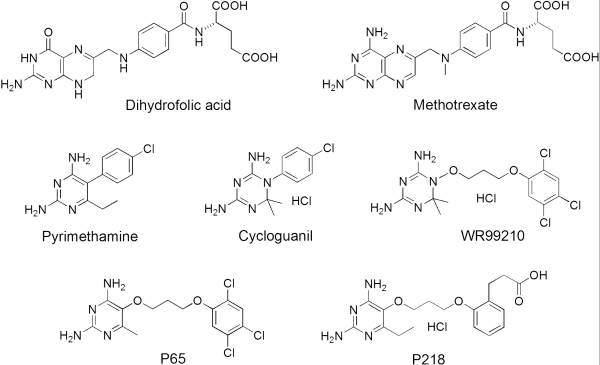
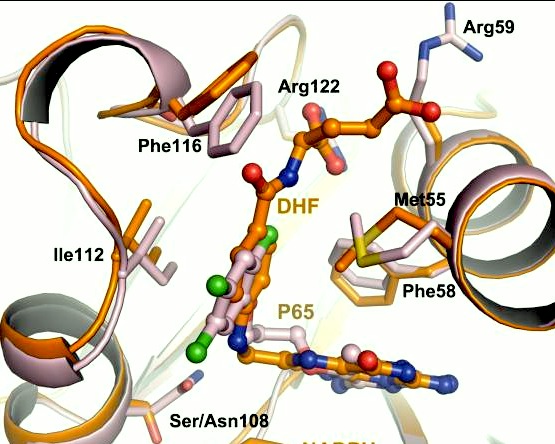
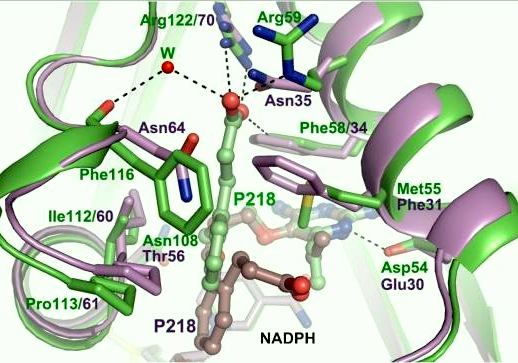
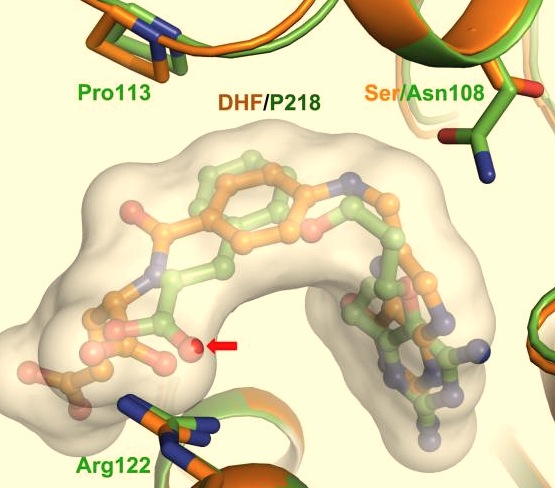
| - | A first step in this direction was to experiment with the drug <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Wr99210/1'>WR99210</scene>, a 4,6-diamino-1,2-dihydro-1,3,5-triazine that, with its (2,4,5-trichlorophenoxy)propoxy side chain, addressed the steric clash that <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Pyrimethamine_with_wt_pfdhfr/2'>pyrimethamine</scene> was subjected to with a Ser108Asn mutation. However further research with this drug was stopped because of its gastrointestinal toxicity in humans and low bioavailbility.<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> Wr99210 was significantly more basic than pyrimethamine, a pyrimidine and slightly acidic substance to match that of the gastrointestinal track, and thus was not readily absorbed in the intestines. When | + | A first step in this direction was to experiment with the drug <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Wr99210/1'>WR99210</scene>, a 4,6-diamino-1,2-dihydro-1,3,5-triazine that, with its (2,4,5-trichlorophenoxy)propoxy side chain, addressed the steric clash that <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Pyrimethamine_with_wt_pfdhfr/2'>pyrimethamine</scene> was subjected to with a Ser108Asn mutation. However further research with this drug was stopped because of its gastrointestinal toxicity in humans and low bioavailbility.<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> Wr99210, like cycloguanil, was significantly more basic than pyrimethamine, a pyrimidine and slightly acidic substance to match that of the gastrointestinal track, and thus was not readily absorbed in the intestines. When present in the gastrointestinal track, the triazines were fully protonated while the 2,4-diaminopyrimidines were present as a mixture of protonated and unprotonated. To test if protonation affected the bioavailability, a less basic compound with a 2,4-diaminopyrimidine scaffold was synthesized called P65. When it was compared via in vitro and in vivo experiments to Wr99210, P65 was shown to have significantly higher absorption in vivo. Thus, P65 was chosen as the basis for more research and the later discussed drug, P218. It was reasoned that protonation most likely interferes with absorption via passive diffusion and though Wr99210 was effective at getting deep into the mutant PfDHFR active site, its protonated state in the acidic gastrointestinal track would have made it unsuccessful as an oral drug, especially in comparison to its diaminopyrimidine analog, P65. |
Revision as of 21:38, 29 November 2012
Introduction
Dihydrofolate reductase (DHFR) plays an essential role in the formation of DNA by managing folate, an organic molecule that shuttles carbons to enzymes that need them for their reactions. Relevant to this context of DHFR involvement, folate gives its carbons to thymidylate synthase, which then uses these carbons to make thymine bases. After folate has removed its carbon atoms, it is the job of DHFR to recycle it. It does this by transferring hydrogen atoms from NADPH to folate to restore it to its useful, reduced form.[1] DHFR is present is used in all organisms however, each organism makes a lsightly different version. The malaria version of the enzyme has shown to be reliable and one of the best targets for antimalarial drugs. The current antimalarial drugs that target the malarial DHFR include pyrimethamine and cycloguanil. Pyrimethamine is currently used in combination sulfadoxine, a sulfadrug, to treat and prevent malaria but can result in serious side effects including liver damage.[2][3] However, recently the effectiveness of these drugs has decreased because of mutations in the enzyme that have led to drug resistance. Since these mutations are becoming much more prevalent in malaria cases, new research in drug development must now incorporate both the wild-type as well as the quadruple mutant DHFR from the Plasmodium falciparum malarial strain, the most common and lethal of the malaria species.[4]
| |||||||||||
References
- ↑ Goodsell, David. "Dihydrofolate Reductase." RCSB PDB-101. RCSB PDB, Oct. 2002. Web. <http://www.rcsb.org/pdb/101/motm.do?momID=34>.
- ↑ "Pyrimethamine & Sulfadoxine." United States National Library of Medicine, n.d. Web. <http://livertox.nih.gov/PyrimethamineSulfadoxine.htm>.
- ↑ "Pyrimethamine and Sulfadoxine (Oral Route)." Mayo Clinic. Mayo Foundation for Medical Education and Research, 01 Nov. 2011. Web. <http://www.mayoclinic.com/health/drug-information/DR600357>.
- ↑ Somsak V, Uthaipibull C, Prommana P, Srichairatanakool S, Yuthavong Y, Kamchonwongpaisan S. Transgenic Plasmodium parasites stably expressing Plasmodium vivax dihydrofolate reductase-thymidylate synthase as in vitro and in vivo models for antifolate screening. Malar J. 2011 Oct 7;10:291. PMID: 21981896
- ↑ Huang F, Tang L, Yang H, Zhou S, Liu H, Li J, Guo S. Molecular epidemiology of drug resistance markers of Plasmodium falciparum in Yunnan Province, China. Malar J. 2012 Jul 28;11:243. PMID: 22839209
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
Proteopedia Page Contributors and Editors (what is this?)
Mary Smith, Alexander Berchansky, Karsten Theis, Michal Harel