Tutorial:Basic Chemistry Topics
From Proteopedia
| Line 222: | Line 222: | ||
==Covalent Bonds== | ==Covalent Bonds== | ||
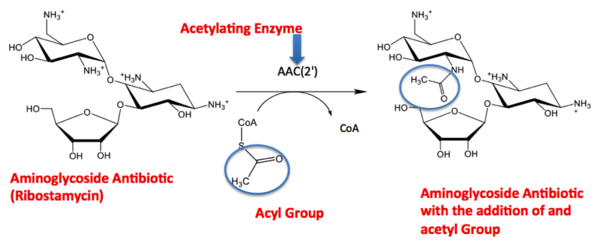
| - | The strongest of these bonds is the covalent bond. Covalent bonds involve sharing pairs of electrons between two atoms. These bonds are very stable. The figure shown below | + | The strongest of these bonds is the covalent bond. Covalent bonds involve sharing pairs of electrons between two atoms. These bonds are very stable. The figure shown below represents Coenzyme A (CoA) with the addition of an acyl group to the sulfur atom. The introduction mentioned that the addition of the acetyl group is important to the study, because when it is transferred to Tobramycin the antibiotic becomes inactive. The solid connections between the atoms are representation of the covalent bonds. |
[[Image:CoA + Acyl group.png | thumb | center | 300px | Covalent bonding]]<ref name="Periodic Table">User:Cepheus. "Periodic Table." Wikipedia. N.p., 26 Feb. 2007. Web. 26 Nov. 2012. <http://en.wikipedia.org/wiki/File:Periodic_table.svg>.</ref> | [[Image:CoA + Acyl group.png | thumb | center | 300px | Covalent bonding]]<ref name="Periodic Table">User:Cepheus. "Periodic Table." Wikipedia. N.p., 26 Feb. 2007. Web. 26 Nov. 2012. <http://en.wikipedia.org/wiki/File:Periodic_table.svg>.</ref> | ||
| Line 230: | Line 230: | ||
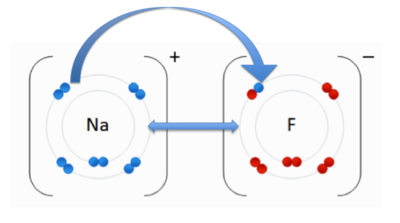
[[Image:Ionic bond.png| thumb | center | 400px | Ionic Bonding<ref>. "File:NaF.gif." Wikipedia. Wikipedia, 17 June 2011. Web. 31 Oct. 2012.<http://en.wikipedia.org/wiki/File:NaF.gif.</ref>]] | [[Image:Ionic bond.png| thumb | center | 400px | Ionic Bonding<ref>. "File:NaF.gif." Wikipedia. Wikipedia, 17 June 2011. Web. 31 Oct. 2012.<http://en.wikipedia.org/wiki/File:NaF.gif.</ref>]] | ||
| - | An ionic bond occurs when an electron(s) is transferred from one atom to another to become more stable with a full electron shell. The charges result from the transfer of an electron. The positively charged cation is attracted to the negatively charged anion. In the image to the right, you see an anion | + | An ionic bond occurs when an electron(s) is transferred from one atom to another to become more stable with a full electron shell. The charges result from the transfer of an electron. The positively charged cation is attracted to the negatively charged anion. In the image to the right, you see an anion Fluoride (F-), and the cation Sodium (Na+). The double-sided arrow between them is representation of their attractive force. Fluorine has a higher electronegativity than sodium. As we discussed previously, when an atom has higher electronegativity it pulls electrons from the lower electronegative atom; in this case sodium. The transfer of the sodium electron (blue) is shown using an arrow. |
Tobramycin and aspartic acid form an <scene name='Tutorial:Basic_Chemistry_Topics/New_ionic_bond/1'>ionic bond</scene> in this representation. The nitrogen on tobramycin has a (+) positive charge and aspartic acid has a (-) negative charge. The opposing charges are attracted to each other forming an ionic bond, holding the compounds in close proximity. | Tobramycin and aspartic acid form an <scene name='Tutorial:Basic_Chemistry_Topics/New_ionic_bond/1'>ionic bond</scene> in this representation. The nitrogen on tobramycin has a (+) positive charge and aspartic acid has a (-) negative charge. The opposing charges are attracted to each other forming an ionic bond, holding the compounds in close proximity. | ||
| Line 237: | Line 237: | ||
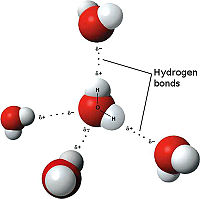
[[Image:3D hydrogen Bonding.jpg | thumb | left | 200px | Hydrogen Bonding<ref>Maňas, Michal, trans. "File:3D model hydrogen bonds in water.jpg." Wikimedia Commons. Wikimedia Commons, 3 Dec. 2007. Web. 31 Oct. 2012 <http://commons.wikimedia.org/wiki/File:3D_model_hydrogen_bonds_in_water.jpg.</ref>]] | [[Image:3D hydrogen Bonding.jpg | thumb | left | 200px | Hydrogen Bonding<ref>Maňas, Michal, trans. "File:3D model hydrogen bonds in water.jpg." Wikimedia Commons. Wikimedia Commons, 3 Dec. 2007. Web. 31 Oct. 2012 <http://commons.wikimedia.org/wiki/File:3D_model_hydrogen_bonds_in_water.jpg.</ref>]] | ||
| - | The weakest bond discussed here, the hydrogen bond is an attractive intermolecular attraction between an electronegative atom and hydrogen. When the electronegative atom pulls the electrons, it leaves the other atom with a slightly positive charge. A common example of this is water. The image to the | + | The weakest bond discussed here, the hydrogen bond is an attractive intermolecular attraction between an electronegative atom and hydrogen. When the electronegative atom pulls the electrons, it leaves the other atom with a slightly positive charge. A common example of this is water. The image to the left demonstrates the hydrogen bonding of water. The highly electronegative oxygen pulls hydrogen's electrons closer. When oxygen pulls the electrons, it leaves hydrogen with a slight positive charge. In this representation the <scene name='Tutorial:Basic_Chemistry_Topics/Hydrogen_bonds/2'>hydrogen bonds</scene> are represented as yellow-dashed lines. The hydrogen bonds are important to the compound used by the study because they offer stability to the secondary structures. |
| Line 257: | Line 257: | ||
='''Secondary Structures'''= | ='''Secondary Structures'''= | ||
| - | Secondary structures are <scene name='Tutorial:Basic_Chemistry_Topics/Alpha_beta_2ndstructures/1'>alpha helices and beta sheets</scene>. The helices and sheets provide stability to the compound as a whole. The alpha helices are represented with pink arrows and the beta sheets are represented with yellow arrows. This molecule has approximately eight alpha helices and four beta sheets. <scene name='Tutorial:Basic_Chemistry_Topics/Alpha_helix/1'>Alpha Helices </scene>have a cylinder-like structure with a parallel formation. In this representation you can see the parallel formation within the cylinder structure. The parallel alpha helices are held in its cylinder structure by hydrogen bonds. <scene name='Tutorial:Basic_Chemistry_Topics/Beta_sheets/1'>Beta sheets</scene> are often anti-parallel. The folding of a protein, alpha helices and beta sheets, gives the compound | + | Secondary structures are <scene name='Tutorial:Basic_Chemistry_Topics/Alpha_beta_2ndstructures/1'>alpha helices and beta sheets</scene>. The helices and sheets provide stability to the compound as a whole. The alpha helices are represented with pink arrows and the beta sheets are represented with yellow arrows. This molecule has approximately eight alpha helices and four beta sheets. <scene name='Tutorial:Basic_Chemistry_Topics/Alpha_helix/1'>Alpha Helices </scene>have a cylinder-like structure with a parallel formation. In this representation you can see the parallel formation within the cylinder structure. The parallel alpha helices are held in its cylinder structure by hydrogen bonds. <scene name='Tutorial:Basic_Chemistry_Topics/Beta_sheets/1'>Beta sheets</scene> are often anti-parallel. The folding of a protein, alpha helices and beta sheets, gives the compound its function. When there is a change in protein folding, the function will change. As previously stated, the study discovered ACC(2’)-Ic to have a GNAT fold, and the GNAT family are enzymes capable of acetylation. |
Revision as of 03:05, 3 December 2012
Purpose of the Tutorial
- This tutorial is intended as a beneficial learning/teaching aid for an entry-level chemistry college student with some basic chemistry knowledge. Various general chemistry concepts are explained using a research article as an example. Applying general chemistry to a research article will allow the students to see the impact they can have on the research world in the future by applying their knowledge.
Summary: Scientific Research Article
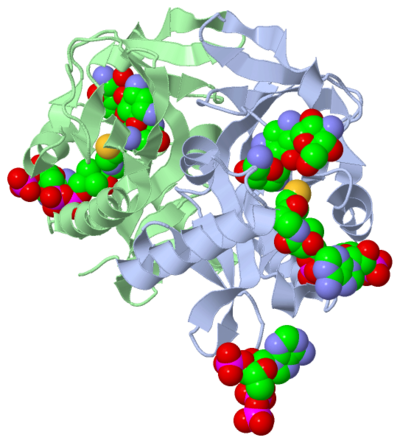
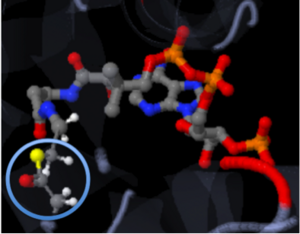
The molecule to left is from the article "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin" published in Nature Structural Biology.[2]. The study focused on aminoglycoside 2’- N- acetyltransferase (AAC (2’)- Ic), an enzyme. This enzyme is a protein that speeds the rate of the reaction it catalyzes.
This study determined the structure of AAC (2’)-Ic from mycobacterium tuberculosis, a pathogen. This pathogen is a microorganism that causes tuberculosis (TB), which typically affects the lungs, but can affect other parts of the body as well. The specific structure/protein fold of AAC (2’)-Ic places it in the GCN5-related N-acetyltransferase (GNAT) superfamily. The GNAT superfamily is a group of enzymes that are similar in structure. The protein fold is important because it determines the function of the compound.[2]
The GNAT family is a group of acetylating enzymes. Acetylation is the addition of CH3CO functional group onto a compound. Although the physiological function of AAC(2’)-Ic is not certain, the discovery of the GNAT fold allowed researchers to classify AAC (2’)-Ic as an acetylating enzyme. Mycothiol is catalyzed by AAC (2’)-Ic to acetylate the aminoglycoside antibiotic, Tobramycin. When this occurs the aminoglycoside antibiotic becomes inactive. The basis of this study is important because when pathogens become resistant to commonly used antibiotics, an infection that was easily cured can now become severe and life threatening.[2]
| |||||||||||
References
- ↑ Vetting, M. W., et al. "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin." RCSB Protien DataBase. N.p., 28 Aug.2002. Web. 13 July 2011. http://www.rcsb.org/pdb/explore/explore.do?structureId=1M4D
- ↑ 2.0 2.1 2.2 2.3 2.4 Vetting, Matthew W., et al. "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin."Nature Structural Biology 9.9 (2002): 653-58. Print.
- ↑ 3.0 3.1 Wikipedia. Wikipedia, 4 Nov. 2012. Web. 7 Nov. 2012. <http://en.wikipedia.org/wiki/Enzyme_substrate_(biology)
- ↑ User:Cepheus. "Periodic Table." Wikipedia. N.p., 26 Feb. 2007. Web. 26 Nov. 2012. <http://en.wikipedia.org/wiki/File:Periodic_table.svg>.
- ↑ . "File:NaF.gif." Wikipedia. Wikipedia, 17 June 2011. Web. 31 Oct. 2012.<http://en.wikipedia.org/wiki/File:NaF.gif.
- ↑ Maňas, Michal, trans. "File:3D model hydrogen bonds in water.jpg." Wikimedia Commons. Wikimedia Commons, 3 Dec. 2007. Web. 31 Oct. 2012 <http://commons.wikimedia.org/wiki/File:3D_model_hydrogen_bonds_in_water.jpg.
- ↑ "Tobramycin." Wikipedia. Wikipedia, n.d. Web. 26 Nov. 2012.<http://en.wikipedia.org/wiki/Tobramycin>.