Tutorial:Basic Chemistry Topics
From Proteopedia
| Line 262: | Line 262: | ||
='''Active Site'''= | ='''Active Site'''= | ||
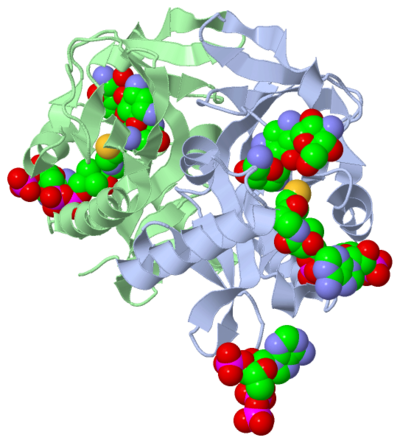
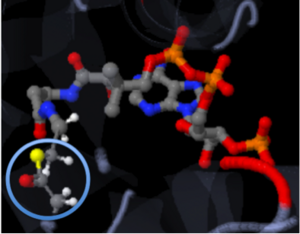
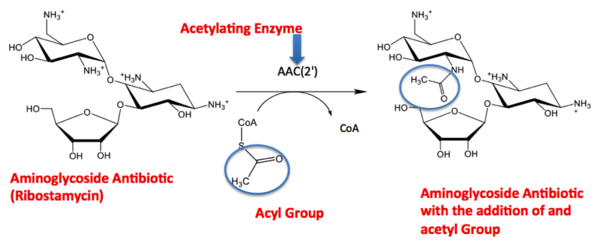
| - | The active site can be described as a pocket where an interaction between complexes produces a chemical reaction. The chemical reaction can be physiological or pathological. Referring back to the article, the active site is where the acetylation is going to occur. In this depiction of the <scene name='Tutorial:Basic_Chemistry_Topics/Active_site/2'>active site</scene> you can see the pocket where coenzyme A (CoA) will aid the enzyme in the acetylation of tobramycin (Toy), the aminoglycoside antibiotic. The enzyme is the "blob" surrounding the antibiotic and CoA. The enzyme is holding these compounds in a conformation forcing them to react. The acetylation at the active site will cause the antibiotic to be inactive, hence inhibiting the active site. When tobramycin becomes inactivated it is no longer able to aid in the destruction of bacteria. This is what we call antibiotic resistance. | + | The active site can be described as a pocket where an interaction between complexes produces a chemical reaction. The chemical reaction can be physiological or pathological. Referring back to the article, the active site is where the acetylation is going to occur. In this depiction of the <scene name='Tutorial:Basic_Chemistry_Topics/Active_site/2'>active site</scene> you can see the pocket where coenzyme A (CoA) will aid the enzyme in the acetylation of tobramycin (Toy), the aminoglycoside antibiotic. The enzyme is the "blob" surrounding the antibiotic and CoA. The enzyme is holding these compounds in a conformation forcing them to react. The acetylation at the active site will cause the antibiotic to be inactive, hence inhibiting the active site. When tobramycin becomes inactivated it is no longer able to aid in the destruction of bacteria. This is what we call "antibiotic resistance". |
| Line 295: | Line 295: | ||
|- | |- | ||
| scope="col" width="5000px" | | | scope="col" width="5000px" | | ||
| - | Tobramycin is an antibiotic which is part of the aminoglycoside family. Aminoglycosides produce antibacterial effects by inhibiting protein synthesis and compromising the structure of the cell wall. By inhibiting protein synthesis of bacteria | + | Tobramycin is an antibiotic which is part of the aminoglycoside family. Aminoglycosides produce antibacterial effects by inhibiting protein synthesis and compromising the structure of the cell wall. By inhibiting protein synthesis of bacteria it prevents the bacteria from replicating. The cell wall is an important structure to bacteria, because it provides the structure and stability to the bacteria. By disrupting the cell wall, the stability of the bacteria is destroyed and ultimately causing bacteria cell death. |
Tobramycin targets a variety of bacteria, particularly gram(-) species. Just like all drugs there are side effects associated with tobramycin. Some of the more common side effects are ototoxicity and nephrotoxicity. Ototoxicity is hearing loss and nephrotoxicity is kidney damage. The kidney damage is due to tobramycin reabsorption through the renal tubules. This basically means that tobramycin may be toxic to the kidneys due to prolonged contact time in the kidneys. | Tobramycin targets a variety of bacteria, particularly gram(-) species. Just like all drugs there are side effects associated with tobramycin. Some of the more common side effects are ototoxicity and nephrotoxicity. Ototoxicity is hearing loss and nephrotoxicity is kidney damage. The kidney damage is due to tobramycin reabsorption through the renal tubules. This basically means that tobramycin may be toxic to the kidneys due to prolonged contact time in the kidneys. | ||
| - | Tobramycin is a pregnancy category D. Pregnancy categories are assigned to all drugs. They are used to classify how likely the drug is to cause harm to the fetus. The pregnancy categories are A, B, C, D, and X. Pregnancy category A causes no harm to the fetus and pregnancy category X | + | Tobramycin is a pregnancy category D. Pregnancy categories are assigned to all drugs. They are used to classify how likely the drug is to cause harm to the fetus. The pregnancy categories are A, B, C, D, and X. Pregnancy category A causes no harm to the fetus and pregnancy category X definitely causes harm to the fetus. Since Tobramycin is a pregnancy category D, this is not an optimal choice for a pregnant patient with a gram(-) bacterial infection. |
| - | Tobramycin can be given intravenously, intramuscularly, as an inhalation or | + | Tobramycin can be given intravenously, intramuscularly, as an inhalation or ophthalmically. Intravenously (IV) is a route of administration where the drug is administered directly to the vasculature or blood vessels. Intramuscular (IM) injection penetrates through the skin to the muscle. A common example of an intramuscular injection is a flu shot. An inhalation delivers the drug directly into the lungs. An example of inhalation drug administration is an inhaler used for asthmatics. Ophthalmic administration is drug administration directly to the eye, such as an eye drop. <ref name="Tobramycin">"Tobramycin." Wikipedia. Wikipedia, n.d. Web. 26 Nov. 2012.<http://en.wikipedia.org/wiki/Tobramycin>.</ref> |
|} | |} | ||
| Line 308: | Line 308: | ||
| - | Drug resistance occurs when microorganisms are no longer vulnerable to an antibiotic. Resistance occurs when a microorganism produces a spontaneous/genetic mutation that protects the bacteria from the drug. Antibiotic resistance to Tobramycin by ''mycobacterium tuberculosis'' is the basis of this study. Antibiotic resistance is a growing public health concern | + | Drug resistance occurs when microorganisms are no longer vulnerable to an antibiotic. Resistance occurs when a microorganism produces a spontaneous/genetic mutation that protects the bacteria from the drug. Antibiotic resistance to Tobramycin by ''mycobacterium tuberculosis'' is the basis of this study. Antibiotic resistance is a growing public health concern. |
| - | There are many causes of drug resistance, including antibiotic overuse and inappropriate antibiotic use. A preventable cause of drug resistance is premature discontinuation of a patient’s antibiotics. Patients tend to discontinue their antibiotics when they start to “feel | + | There are many causes of drug resistance, including antibiotic overuse and inappropriate antibiotic use. A preventable cause of drug resistance is premature discontinuation of a patient’s antibiotics. Patients tend to discontinue their antibiotics when they start to “feel better,” but sometimes the infection is not completely eradicated. In the beginning of an antibiotic regimen it begins by killing the most susceptible microorganisms first. As you complete the antibiotic course, the continuous exposure of the antibiotic is able to kill the few more resistant microorganisms. When the antibiotic is discontinued early, the more resistant bacteria are not eliminated. The resistant strain of bacteria will replicate/form resistance and the same antibiotic will not be as effective anymore. |
When drug resistance occurs, the drug/antibiotic needs to be modified to prevent its inactivation. In this study tobramycin is acetylated, which causes it inactivation. If the acetylation of tobramycin can be prevented, tobramycin can remain active and continue its antibacterial effects. One possible theory for inhibiting tobramycin’s acetylation is the addition of a bulky substituent near the active site. Adding a bulky substituent to tobramycin would “block” the addition of the acetyl group, because the acetyl group cannot get to the site of action. The bulky substituent is too large and the acetyl group is not able to fit anymore. There are many things that can be accomplished with the understanding of basic chemistry; there will always be a demand for a brilliant chemist. | When drug resistance occurs, the drug/antibiotic needs to be modified to prevent its inactivation. In this study tobramycin is acetylated, which causes it inactivation. If the acetylation of tobramycin can be prevented, tobramycin can remain active and continue its antibacterial effects. One possible theory for inhibiting tobramycin’s acetylation is the addition of a bulky substituent near the active site. Adding a bulky substituent to tobramycin would “block” the addition of the acetyl group, because the acetyl group cannot get to the site of action. The bulky substituent is too large and the acetyl group is not able to fit anymore. There are many things that can be accomplished with the understanding of basic chemistry; there will always be a demand for a brilliant chemist. | ||
Revision as of 03:30, 3 December 2012
Purpose of the Tutorial
- This tutorial is intended as a beneficial learning/teaching aid for an entry-level chemistry college student with some basic chemistry knowledge. Various general chemistry concepts are explained using a research article as an example. Applying general chemistry to a research article will allow the students to see the impact they can have on the research world in the future by applying their knowledge.
Summary: Scientific Research Article
The molecule to left is from the article "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin" published in Nature Structural Biology.[2]. The study focused on aminoglycoside 2’- N- acetyltransferase (AAC (2’)- Ic), an enzyme. This enzyme is a protein that speeds the rate of the reaction it catalyzes.
This study determined the structure of AAC (2’)-Ic from mycobacterium tuberculosis, a pathogen. This pathogen is a microorganism that causes tuberculosis (TB), which typically affects the lungs, but can affect other parts of the body as well. The specific structure/protein fold of AAC (2’)-Ic places it in the GCN5-related N-acetyltransferase (GNAT) superfamily. The GNAT superfamily is a group of enzymes that are similar in structure. The protein fold is important because it determines the function of the compound.[2]
The GNAT family is a group of acetylating enzymes. Acetylation is the addition of CH3CO functional group onto a compound. Although the physiological function of AAC(2’)-Ic is not certain, the discovery of the GNAT fold allowed researchers to classify AAC (2’)-Ic as an acetylating enzyme. Mycothiol is catalyzed by AAC (2’)-Ic to acetylate the aminoglycoside antibiotic known as tobramycin. When this occurs the aminoglycoside antibiotic becomes inactive. The basis of this study is important because when pathogens become resistant to commonly used antibiotics, an infection that was easily cured can now become severe and life threatening.[2]
| |||||||||||
References
- ↑ Vetting, M. W., et al. "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin." RCSB Protien DataBase. N.p., 28 Aug.2002. Web. 13 July 2011. http://www.rcsb.org/pdb/explore/explore.do?structureId=1M4D
- ↑ 2.0 2.1 2.2 2.3 2.4 Vetting, Matthew W., et al. "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin."Nature Structural Biology 9.9 (2002): 653-58. Print.
- ↑ 3.0 3.1 Wikipedia. Wikipedia, 4 Nov. 2012. Web. 7 Nov. 2012. <http://en.wikipedia.org/wiki/Enzyme_substrate_(biology)
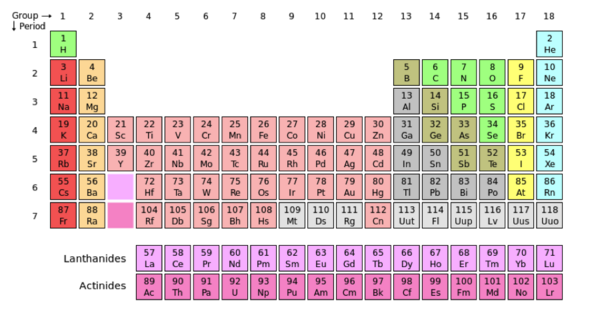
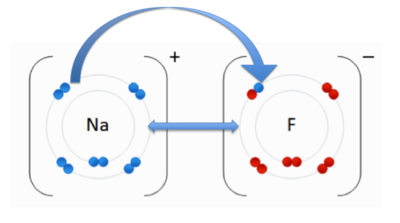
- ↑ User:Cepheus. "Periodic Table." Wikipedia. N.p., 26 Feb. 2007. Web. 26 Nov. 2012. <http://en.wikipedia.org/wiki/File:Periodic_table.svg>.
- ↑ . "File:NaF.gif." Wikipedia. Wikipedia, 17 June 2011. Web. 31 Oct. 2012.<http://en.wikipedia.org/wiki/File:NaF.gif.
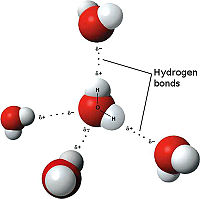
- ↑ Maňas, Michal, trans. "File:3D model hydrogen bonds in water.jpg." Wikimedia Commons. Wikimedia Commons, 3 Dec. 2007. Web. 31 Oct. 2012 <http://commons.wikimedia.org/wiki/File:3D_model_hydrogen_bonds_in_water.jpg.
- ↑ "Tobramycin." Wikipedia. Wikipedia, n.d. Web. 26 Nov. 2012.<http://en.wikipedia.org/wiki/Tobramycin>.