We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 390
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
__TOC__ | __TOC__ | ||
==INTRODUCTION == | ==INTRODUCTION == | ||
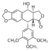
| - | The research team led by professor Nei-li Chan and Tsai-kun Li at College of Medicine, National Taiwan University described the structural basis by which an anticancer drug etoposide kills cancer cells by interacting with its cellular targets human DNA topoisomerase type II <ref name=”two”> PMID: 21778401</ref>. In the close-up representation of the <scene name='Sandbox_Reserved_390/Top/21'>etoposide-binding site(s),</scene> cartoon-and-stick representation shows the insertion of two etoposide molecules into two cleavage sites [etoposide surrounded by orange mesh that represent active site (etoposide in red & grey representation), the DNA chain is in red and blue, and the magnesium is in green]. The neighboring magnesium’s are believed to be use at both the ATP binding domain and in the cleavage core. In the nucleotide binding pocket magnesium is known to contact all of the phosphate groups and it’s possible that the changes in magnesium can be linked to a diminishing in the nucleotide-binding pocket. | + | The research team led by professor Nei-li Chan and Tsai-kun Li at College of Medicine, National Taiwan University described the structural basis by which an anticancer drug etoposide kills cancer cells by interacting with its cellular targets human DNA topoisomerase type II <ref name=”two”> Wu CC, Li TK, Farh L, Lin LY, Lin TS, Yu YJ, Yen TJ, Chiang CW, Chan NL. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science. 2011 Jul 22;333(6041):459-62. PMID: 21778401</ref>. In the close-up representation of the <scene name='Sandbox_Reserved_390/Top/21'>etoposide-binding site(s),</scene> cartoon-and-stick representation shows the insertion of two etoposide molecules into two cleavage sites [etoposide surrounded by orange mesh that represent active site (etoposide in red & grey representation), the DNA chain is in red and blue, and the magnesium is in green]. The neighboring magnesium’s are believed to be use at both the ATP binding domain and in the cleavage core. In the nucleotide binding pocket magnesium is known to contact all of the phosphate groups and it’s possible that the changes in magnesium can be linked to a diminishing in the nucleotide-binding pocket. |
==ENZYME== | ==ENZYME== | ||
Type II topoisomerases (TOP2s) are abundant enzymes that play an essential role in <scene name='Sandbox_Reserved_390/Top/5'>DNA</scene> replication and transcription and are important targets for cancer chemotherapeutic drugs. These enzymes briefly cleave a pair of opposing phosphodiester bonds four base pairs apart, generating a TOP2-DNA cleavage complex. | Type II topoisomerases (TOP2s) are abundant enzymes that play an essential role in <scene name='Sandbox_Reserved_390/Top/5'>DNA</scene> replication and transcription and are important targets for cancer chemotherapeutic drugs. These enzymes briefly cleave a pair of opposing phosphodiester bonds four base pairs apart, generating a TOP2-DNA cleavage complex. | ||
Revision as of 14:45, 3 December 2012
Human topoisomerase II beta in complex with DNA and etoposide
| |||||||||||
References
- ↑ Wu CC, Li TK, Farh L, Lin LY, Lin TS, Yu YJ, Yen TJ, Chiang CW, Chan NL. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science. 2011 Jul 22;333(6041):459-62. PMID: 21778401
- ↑ Kathryn L. Gilroy, Chrysoula Leontiou, Kay Padget, Jeremy H. Lakey and Caroline A. Austin* "mAMSA resistant human topoisomerase IIβ mutation G465D has reduced ATP hydrolysis activity” Oxford JournalsLife Sciences Nucleic Acids Research Volume 34, Issue 5Pp. 1597-1607. DOI: 10.1093/nar/gkl057
- ↑ Sorrentino BP. Gene therapy to protect haematopoietic cells from cytotoxic cancer drugs. Nat Rev Cancer. 2002 Jun;2(6):431-41. PMID:12189385 doi:10.1038/nrc823