We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 390
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
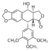
TOP2’s DNA cleavage activity is usually referred to as a double-edged sword; failure to reseal the enzyme-mediated DNA break can lead to cell death. Several potent anticancer drugs, such as <scene name='Sandbox_Reserved_390/Etoposide/1'>etoposide</scene> , <scene name='Sandbox_Reserved_390/Doxorubicin/1'>doxorubicin</scene> and <scene name='Sandbox_Reserved_390/Mitoxantron/1'>mitoxantrone</scene> (all in green), exploit this harmful aspect of TOP2 and promote the formation of cytotoxic DNA lesions by increasing the stability level of cleavage complexes. <ref> Kathryn L. Gilroy, Chrysoula Leontiou, Kay Padget, Jeremy H. Lakey and Caroline A. Austin* "mAMSA resistant human topoisomerase IIβ mutation G465D has reduced ATP hydrolysis activity” Oxford JournalsLife Sciences Nucleic Acids Research Volume 34, Issue 5Pp. 1597-1607. [http://nar.oxfordjournals.org/content/34/5/1597 DOI: 10.1093/nar/gkl057]</ref> | TOP2’s DNA cleavage activity is usually referred to as a double-edged sword; failure to reseal the enzyme-mediated DNA break can lead to cell death. Several potent anticancer drugs, such as <scene name='Sandbox_Reserved_390/Etoposide/1'>etoposide</scene> , <scene name='Sandbox_Reserved_390/Doxorubicin/1'>doxorubicin</scene> and <scene name='Sandbox_Reserved_390/Mitoxantron/1'>mitoxantrone</scene> (all in green), exploit this harmful aspect of TOP2 and promote the formation of cytotoxic DNA lesions by increasing the stability level of cleavage complexes. <ref> Kathryn L. Gilroy, Chrysoula Leontiou, Kay Padget, Jeremy H. Lakey and Caroline A. Austin* "mAMSA resistant human topoisomerase IIβ mutation G465D has reduced ATP hydrolysis activity” Oxford JournalsLife Sciences Nucleic Acids Research Volume 34, Issue 5Pp. 1597-1607. [http://nar.oxfordjournals.org/content/34/5/1597 DOI: 10.1093/nar/gkl057]</ref> | ||
| - | In this paper, the researchers reported on the crystal structure of a large fragment of type II human topoisomerases β (hTOP2β core) complexed to DNA and to the anticancer drug etoposide to reveal structural details of drug-induced stabilization of a cleavage complex <ref | + | In this paper, the researchers reported on the crystal structure of a large fragment of type II human topoisomerases β (hTOP2β core) complexed to DNA and to the anticancer drug etoposide to reveal structural details of drug-induced stabilization of a cleavage complex <ref>PMID: 21778401</ref>. This structure provided the first observation of a TOP2 ternary cleavage complex <scene name='Sandbox_Reserved_390/Top/22'>stabilized</scene> by an anticancer drug. |
The high-resolution structure of the hTOP2βcore-DNA-etoposide ternary complex reveals the intricate interplays between <scene name='Sandbox_Reserved_390/Top/6'>protein</scene>, <scene name='Sandbox_Reserved_390/Top/16'>DNA</scene> and <scene name='Sandbox_Reserved_390/Top/23'>drugs</scene>. This aspect is extremely important because all vertebrates possess two highly similar yet functionally distinct TOP2 isoforms. The α-isoform is particularly important for DNA replication and is usually present at high levels in fast growing cancer cells, whereas the β-isoform is mainly involved in transcription related processes. Although the inhibition of both TOP2 isoforms contributes to the drug-induced death of cancer cells, targeting of the β-isoform has been implicated in deleterious therapy related secondary malignancies. Therefore, it is desirable to develop the isoform specific TOP2-targeting agents. | The high-resolution structure of the hTOP2βcore-DNA-etoposide ternary complex reveals the intricate interplays between <scene name='Sandbox_Reserved_390/Top/6'>protein</scene>, <scene name='Sandbox_Reserved_390/Top/16'>DNA</scene> and <scene name='Sandbox_Reserved_390/Top/23'>drugs</scene>. This aspect is extremely important because all vertebrates possess two highly similar yet functionally distinct TOP2 isoforms. The α-isoform is particularly important for DNA replication and is usually present at high levels in fast growing cancer cells, whereas the β-isoform is mainly involved in transcription related processes. Although the inhibition of both TOP2 isoforms contributes to the drug-induced death of cancer cells, targeting of the β-isoform has been implicated in deleterious therapy related secondary malignancies. Therefore, it is desirable to develop the isoform specific TOP2-targeting agents. | ||
Current revision
Human topoisomerase II beta in complex with DNA and etoposide
| |||||||||||
References
- ↑ Wu CC, Li TK, Farh L, Lin LY, Lin TS, Yu YJ, Yen TJ, Chiang CW, Chan NL. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science. 2011 Jul 22;333(6041):459-62. PMID:21778401 doi:10.1126/science.1204117
- ↑ Kathryn L. Gilroy, Chrysoula Leontiou, Kay Padget, Jeremy H. Lakey and Caroline A. Austin* "mAMSA resistant human topoisomerase IIβ mutation G465D has reduced ATP hydrolysis activity” Oxford JournalsLife Sciences Nucleic Acids Research Volume 34, Issue 5Pp. 1597-1607. DOI: 10.1093/nar/gkl057
- ↑ Wu CC, Li TK, Farh L, Lin LY, Lin TS, Yu YJ, Yen TJ, Chiang CW, Chan NL. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science. 2011 Jul 22;333(6041):459-62. PMID:21778401 doi:10.1126/science.1204117
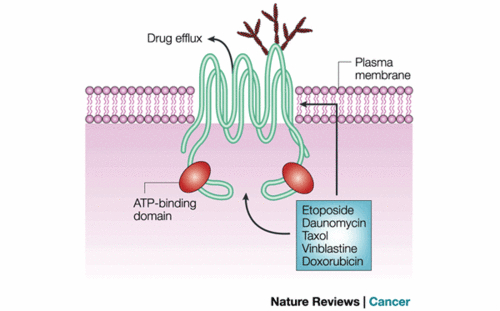
- ↑ Sorrentino BP. Gene therapy to protect haematopoietic cells from cytotoxic cancer drugs. Nat Rev Cancer. 2002 Jun;2(6):431-41. PMID:12189385 doi:10.1038/nrc823