Group:MUZIC:Myozenin
From Proteopedia

(→Pathology) |
(→Pathology) |
||
| Line 18: | Line 18: | ||
It was shown that FATZ-2 KO mouse hearts subjected to pressure overload chronically activated the calcineurin/NFAT signalling pathway. In consequence, those animals showed hypertrophic hearts and developed cardiomyopathy. Moreover, the transgenic over-expression of FATZ-2 rescued the mouse hearts from angiotensin II-induced cardiac hypertrophy, which suggested that FATZ-2 could be a target gene affected in patients with the same disease <ref>PMID: 15543153</ref>. | It was shown that FATZ-2 KO mouse hearts subjected to pressure overload chronically activated the calcineurin/NFAT signalling pathway. In consequence, those animals showed hypertrophic hearts and developed cardiomyopathy. Moreover, the transgenic over-expression of FATZ-2 rescued the mouse hearts from angiotensin II-induced cardiac hypertrophy, which suggested that FATZ-2 could be a target gene affected in patients with the same disease <ref>PMID: 15543153</ref>. | ||
| - | The study of two families with clinical symptoms of hypertrophic cardiomyopathy(HCM) suggested that mutations S48P and I246M in FATZ-2 where associated with familial HCM <ref>PMID: 17347475 </ref>. However, another study of 438 patiens concluded that mutations in FATZ-2 were rare causes of familial HCM. <ref>PMID: 18591919</ref>. | + | The study of two families with clinical symptoms of hypertrophic cardiomyopathy(HCM) suggested that mutations S48P and I246M in FATZ-2 where associated with familial HCM <ref>PMID: 17347475 </ref>. However, another study of 438 patiens concluded that mutations in FATZ-2 were rare causes of familial HCM. <ref>PMID: 18591919</ref>. Recently experiments in mice models expressing the mutants FATZ-2 S48P and I246M showed the clinical symptoms of HCM, myofibrillar disarray and Z-disc structural abnormalities. However, no upregulation of the calcineurin/NFAT signalling pathway was observed, suggesting that HCM caused by those mutations is not associated to the inhibitory effect of FATZ-2 on calcineurin. <ref>PMID: 22987565</ref>. |
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 10:32, 12 December 2012
Contents |
Introduction
The filamin-C α-actinin telethonin Z-disc binding protein (FATZ) is a protein family of three isoforms: FATZ-1, FATZ-2, FATZ-3, which are expressed in muscle cells.[1] This protein family, that is also known as Myozenin or Calsarcin, is mainly localized in the Z-disc, although recently it has been described that FATZ-2 appears in cardiac nuclei. [2] The expression of the three isoforms has been shown to be fibre type specific. For instance, FATZ-1 and FATZ-3 are exclusively expressed in skeletal muscle fast-twitch fibres while FATZ-2 is expressed in cardiac and slow-twitch fibres [3][4]. FATZ proteins have multiple binding partners in the Z-disc, which involve them in different functions like the Z-disc formation and maintenance or in signaling pathways like the calcineurin/NFAT [5]. Therefore, the FATZ protein family could be seen as one example of Z-disc proteins where signalling and structural support converge.
Sequence Annotation
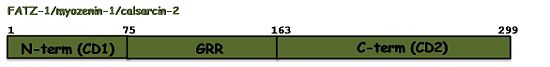
The sequence annotation of FATZ-1 is related to the sequence conservation profile among the three isoforms, which share well conserved N-terminal and C-terminal regions. Therefore, the N-terminal of FATZ-1 was named conserved domain 1 (CD1, 1-75aa) and its C-terminal conserved domain 2 (CD2, 172-299aa). Both regions are connected by a stretch of amino acids with a 39.5% of glycine; consequently, this region was named the glycine rich region (GRR, 75-171aa)[6] Q9NP98. Although the N-terminal and C-terminal regions of the other two isoforms could be also named CD1 and CD2; no such sequence annotations exist in their UniProtKB entries Q9NPC6Q8TDC0.Function and Interactions
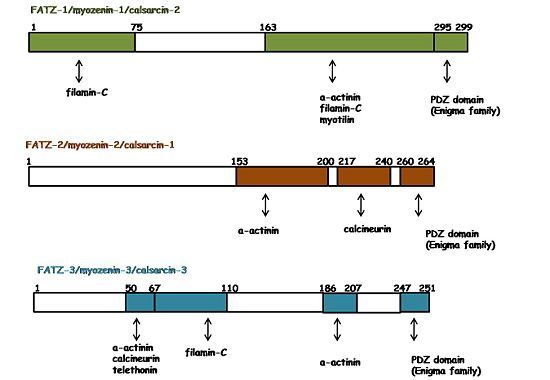
The three isoforms of FATZ have a plethora of interacting partners which are additionally shared by all of them. As shown by the figure on the right, they interact with several Z-disc proteins. For instance: α-actinin-2, filamin-C, myotilin, telethonin, calcineurin and ZASP/Cypher (in general the Enigma protein family) [7][8][9][10][11][12]. In general, those interactions and their binding regions were found by yeast two-hybrid assays, co-immunoprecipitation, and pull down assays.The interaction of FATZ-1 with α-actinin-2 was reported simultaneously by three different groups [13][14][15]. It was documented with the three isoforms of FATZ, which showed their binding regions within the CD2. Furthermore, the data suggested a binding interface on α-actinin-2 starting in the middle of the SR2, followed by the SR3 and the SR4. FATZ-1, α-actinin-2 and myotilin appeared in premyofibrils, when there is no Z-disc but a small structure called Z-body. In addition, the complexes FATZ-1::α-actinin-2, FATZ-1::myotilin and myotilin::α-actinin-2 were also observed in that early stages. In contrast, telethonin localized to the Z-disc in later stages and the complex FATZ-1::telethonin was only observed in mature myofibrils. These findings suggested that the interactions of FATZ-1 with α-actinin-2 and myotilin are very important for the initiation of the Z-disc assembling. Besides, it was hypothesized that FATZ-1 should undergone conformational changes during myofibrilogenesis that determines the interaction with telethonin; therefore, its incorporation to the the Z-disc [16]. FATZ-1 has been suggested to perform other functions, like bridging filamin-C and α-actinin-2. However, it was reported in a competitive binding assay that α-actinin-2 displaces filamin-C form FATZ-1. Therefore, it is still an open question whether a ternary complex could exist and what its physiological role would be [17].
Both isoforms, FATZ-1 and FATZ-2 were shown to be negative regulators of the calcineurin/NFAT signaling pathway in striated muscle. In skeletal muscle, the activation of calcineurin/NFAT specifically determines the switch to the phenotype slow-twitch fibers. FATZ-1 is specifically expressed in fast-twitch fibers and its absence was seen to switch the skeletal muscle composition toward slow-twitch fiber0. This was associated with an increase in the activity of the calcineurin/NFAT signaling pathway, consequently a major number of oxidative fibers, and less fatigue of the mutant vs the wild type mice during long endurance exercise . Therefore, it was concluded that FATZ-1 interaction with calcineurin defines the fiber type composition of skeletal muscle and the response to exercise performance [18]. The isoform FATZ-2 also controls the activity of calcineurin in cardiac fibers; therefore, it is an important protein to maintain the homeostasis of cardiac fibers subjected to pressure overload via the calcineurin/NFAT signalling pathway [19].
Pathology
It was shown that FATZ-2 KO mouse hearts subjected to pressure overload chronically activated the calcineurin/NFAT signalling pathway. In consequence, those animals showed hypertrophic hearts and developed cardiomyopathy. Moreover, the transgenic over-expression of FATZ-2 rescued the mouse hearts from angiotensin II-induced cardiac hypertrophy, which suggested that FATZ-2 could be a target gene affected in patients with the same disease [20]. The study of two families with clinical symptoms of hypertrophic cardiomyopathy(HCM) suggested that mutations S48P and I246M in FATZ-2 where associated with familial HCM [21]. However, another study of 438 patiens concluded that mutations in FATZ-2 were rare causes of familial HCM. [22]. Recently experiments in mice models expressing the mutants FATZ-2 S48P and I246M showed the clinical symptoms of HCM, myofibrillar disarray and Z-disc structural abnormalities. However, no upregulation of the calcineurin/NFAT signalling pathway was observed, suggesting that HCM caused by those mutations is not associated to the inhibitory effect of FATZ-2 on calcineurin. [23].
References
- ↑ Faulkner G, Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C, Trevisan S, Kojic' S, Dalla Vecchia F, Laveder P, Valle G, Lanfranchi G. FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle. J Biol Chem. 2000 Dec 29;275(52):41234-42. PMID:10984498 doi:10.1074/jbc.M007493200
- ↑ Paulsson AK, Franklin S, Mitchell-Jordan SA, Ren S, Wang Y, Vondriska TM. Post-translational regulation of calsarcin-1 during pressure overload-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010 Jun;48(6):1206-14. Epub 2010 Feb 17. PMID:20170660 doi:10.1016/j.yjmcc.2010.02.009
- ↑ Frey N, Richardson JA, Olson EN. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14632-7. PMID:11114196 doi:10.1073/pnas.260501097
- ↑ Frey N, Olson EN. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J Biol Chem. 2002 Apr 19;277(16):13998-4004. Epub 2002 Feb 12. PMID:11842093 doi:10.1074/jbc.M200712200
- ↑ Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA, Olson EN. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med. 2004 Dec;10(12):1336-43. Epub 2004 Nov 14. PMID:15543153 doi:nm1132
- ↑ Takada F, Vander Woude DL, Tong HQ, Thompson TG, Watkins SC, Kunkel LM, Beggs AH. Myozenin: an alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1595-600. Epub 2001 Feb 6. PMID:11171996 doi:10.1073/pnas.041609698
- ↑ Takada F, Vander Woude DL, Tong HQ, Thompson TG, Watkins SC, Kunkel LM, Beggs AH. Myozenin: an alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1595-600. Epub 2001 Feb 6. PMID:11171996 doi:10.1073/pnas.041609698
- ↑ Frey N, Olson EN. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J Biol Chem. 2002 Apr 19;277(16):13998-4004. Epub 2002 Feb 12. PMID:11842093 doi:10.1074/jbc.M200712200
- ↑ von Nandelstadh P, Ismail M, Gardin C, Suila H, Zara I, Belgrano A, Valle G, Carpen O, Faulkner G. A class III PDZ binding motif in the myotilin and FATZ families binds enigma family proteins: a common link for Z-disc myopathies. Mol Cell Biol. 2009 Feb;29(3):822-34. Epub 2008 Dec 1. PMID:19047374 doi:10.1128/MCB.01454-08
- ↑ Gontier Y, Taivainen A, Fontao L, Sonnenberg A, van der Flier A, Carpen O, Faulkner G, Borradori L. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J Cell Sci. 2005 Aug 15;118(Pt 16):3739-49. Epub 2005 Aug 2. PMID:16076904 doi:10.1242/jcs.02484
- ↑ Frey N, Richardson JA, Olson EN. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14632-7. PMID:11114196 doi:10.1073/pnas.260501097
- ↑ Faulkner G, Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C, Trevisan S, Kojic' S, Dalla Vecchia F, Laveder P, Valle G, Lanfranchi G. FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle. J Biol Chem. 2000 Dec 29;275(52):41234-42. PMID:10984498 doi:10.1074/jbc.M007493200
- ↑ Faulkner G, Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C, Trevisan S, Kojic' S, Dalla Vecchia F, Laveder P, Valle G, Lanfranchi G. FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle. J Biol Chem. 2000 Dec 29;275(52):41234-42. PMID:10984498 doi:10.1074/jbc.M007493200
- ↑ Takada F, Vander Woude DL, Tong HQ, Thompson TG, Watkins SC, Kunkel LM, Beggs AH. Myozenin: an alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1595-600. Epub 2001 Feb 6. PMID:11171996 doi:10.1073/pnas.041609698
- ↑ Frey N, Olson EN. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J Biol Chem. 2002 Apr 19;277(16):13998-4004. Epub 2002 Feb 12. PMID:11842093 doi:10.1074/jbc.M200712200
- ↑ Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton. 2005 May;61(1):34-48. PMID:15810059 doi:10.1002/cm.20063
- ↑ Takada F, Vander Woude DL, Tong HQ, Thompson TG, Watkins SC, Kunkel LM, Beggs AH. Myozenin: an alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1595-600. Epub 2001 Feb 6. PMID:11171996 doi:10.1073/pnas.041609698
- ↑ Frey N, Frank D, Lippl S, Kuhn C, Kogler H, Barrientos T, Rohr C, Will R, Muller OJ, Weiler H, Bassel-Duby R, Katus HA, Olson EN. Calsarcin-2 deficiency increases exercise capacity in mice through calcineurin/NFAT activation. J Clin Invest. 2008 Nov;118(11):3598-608. doi: 10.1172/JCI36277. Epub 2008 Oct 9. PMID:18846255 doi:10.1172/JCI36277
- ↑ Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA, Olson EN. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med. 2004 Dec;10(12):1336-43. Epub 2004 Nov 14. PMID:15543153 doi:nm1132
- ↑ Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA, Olson EN. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med. 2004 Dec;10(12):1336-43. Epub 2004 Nov 14. PMID:15543153 doi:nm1132
- ↑ Osio A, Tan L, Chen SN, Lombardi R, Nagueh SF, Shete S, Roberts R, Willerson JT, Marian AJ. Myozenin 2 is a novel gene for human hypertrophic cardiomyopathy. Circ Res. 2007 Mar 30;100(6):766-8. Epub 2007 Mar 8. PMID:17347475 doi:10.1161/01.RES.0000263008.66799.aa
- ↑ Posch MG, Thiemann L, Tomasov P, Veselka J, Cardim N, Garcia-Castro M, Coto E, Perrot A, Geier C, Dietz R, Haverkamp W, Ozcelik C. Sequence analysis of myozenin 2 in 438 European patients with familial hypertrophic cardiomyopathy. Med Sci Monit. 2008 Jul;14(7):CR372-4. PMID:18591919
- ↑ Ruggiero A, Chen SN, Lombardi R, Rodriguez G, Marian AJ. Pathogenesis of hypertrophic cardiomyopathy caused by myozenin 2 mutations is independent of calcineurin activity. Cardiovasc Res. 2012 Oct 19. PMID:22987565 doi:10.1093/cvr/cvs294