Caspase-3 Regulatory Mechanisms
From Proteopedia
| Line 11: | Line 11: | ||
== Structure of Caspase-3 == | == Structure of Caspase-3 == | ||
| + | [[Image:Schematic.png | thumb | Procaspase-3 / Zymogen ]] | ||
Caspase-3 shares many structural characteristics with other caspases. It is synthesized in the cell in its zymogen form, consisting of an N-terminal prodomain followed by a <scene name='Sandox_Bay_Serrano/Dimer_gray/1'>large and small subunit</scene> linked to each other by an intersubunit linker. As an executioner caspase, caspase-3 has a short N-terminal prodomain and like any other caspases, cleavage of the intersubunit linker at a specific aspartate residue generates the mature form of the enzyme, consisting of the large (p17) and small (p12) subunit. Caspase-3 in its functional form is dimeric, with the dimer interface being stabilized by interactions between the small subunits of each monomer. ß-sheets from each monomer interact via hydrophobic interactions resulting in a 12-stranded <scene name='Sandox_Bay_Serrano/Scene01_dimer/2'>ß-sheet structure</scene>, around which α-helices are positioned. | Caspase-3 shares many structural characteristics with other caspases. It is synthesized in the cell in its zymogen form, consisting of an N-terminal prodomain followed by a <scene name='Sandox_Bay_Serrano/Dimer_gray/1'>large and small subunit</scene> linked to each other by an intersubunit linker. As an executioner caspase, caspase-3 has a short N-terminal prodomain and like any other caspases, cleavage of the intersubunit linker at a specific aspartate residue generates the mature form of the enzyme, consisting of the large (p17) and small (p12) subunit. Caspase-3 in its functional form is dimeric, with the dimer interface being stabilized by interactions between the small subunits of each monomer. ß-sheets from each monomer interact via hydrophobic interactions resulting in a 12-stranded <scene name='Sandox_Bay_Serrano/Scene01_dimer/2'>ß-sheet structure</scene>, around which α-helices are positioned. | ||
Revision as of 00:24, 13 December 2012
Introduction
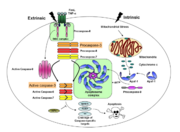
Caspases are cysteine-aspartic acid proteases and are key protein facilitators for the faithful execution of apoptosis or programmed cell death. Dysregulation in the apoptotic pathway has been implicated in a variety of diseases such as neurodegeneration, cancer, heart disease and some metabolic disorders. Because of the crucial role of caspases in the the apoptotic pathway, abnormalities in their functions would cause a haywire in the apoptotic cascade and can be deleterious to the cell. Caspases are thus being considered as therapeutic targets in apoptosis-related diseases.
Any apoptotic signal received by the cell causes the activation of initiator caspases (-8 and -9) by associating with another protein platform to form a functional holoenzyme. These initiator caspases then cleaves the executioner caspases -3, -6, -7. Caspase-3 specifically functions to cleave both caspase-6 and -7, which in turn cleave their respective targets to induce cell death. Aside from being able to activate caspase-6 and -7, caspase-3 also regulates caspase-9 activity, operating via feedback loop. These dual action of caspase-3 confers its distinct regulatory mechanisms, resulting a wider extent of effects in the apoptotic cascade.
| |||||||||||
Caspase-3 Regulation
| |||||||||||
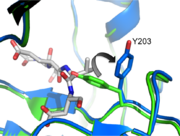
Caspase-3 Loop Bundle and Active Site
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Scott Eron, Banyuhay P. Serrano, Alexander Berchansky, Yunlong Zhao, Jaime Prilusky, Michal Harel