Caspase-3 Regulatory Mechanisms
From Proteopedia
(→Caspase-3 Loop Bundle and Active Site) |
|||
| Line 35: | Line 35: | ||
The active site of caspase-3 utilizes a cysteine-histidine dyad, which has an exquisite specificity for cleaving after aspartate residues. Therefore, caspase-3, by definition, will have an aspartate in the <scene name='Caspase-3_Regulatory_Mechanisms/P1/2'>P1</scene> pocket. Uncleavable peptide substrates are often used in crystallography to bind to the active site. This will orient the delicate but deadly active site loops in order to facilitate the visualization of the chemistry of cleavage. The nucleophilic Cysteine 163 will work in concert with the second active site residue, Histidine 121, to attack the substrate. This reaction will ultimately cleave the peptide bond following the aspartate. | The active site of caspase-3 utilizes a cysteine-histidine dyad, which has an exquisite specificity for cleaving after aspartate residues. Therefore, caspase-3, by definition, will have an aspartate in the <scene name='Caspase-3_Regulatory_Mechanisms/P1/2'>P1</scene> pocket. Uncleavable peptide substrates are often used in crystallography to bind to the active site. This will orient the delicate but deadly active site loops in order to facilitate the visualization of the chemistry of cleavage. The nucleophilic Cysteine 163 will work in concert with the second active site residue, Histidine 121, to attack the substrate. This reaction will ultimately cleave the peptide bond following the aspartate. | ||
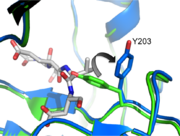
| - | In order to be active and cleave | + | In order to be active and cleave specific apoptotic targets, Caspase-3 must be able to first bind substrate. There are several essential interactions responsible for securing the substrate before cleavage. The binding pocket at <scene name='Caspase-3_Regulatory_Mechanisms/P2/2'>P2</scene> is a hydrophobic patch made up of Y204, W206, and F250 (dark blue residues). This creates a hydrophobic pocket for the P2 residue (in this case, valine), helping it stick to the protein. At <scene name='Caspase-3_Regulatory_Mechanisms/P4/2'>P4</scene> there are contacts that contribute to the specificity of caspase-3. Asparagine 208 hydrogen bonds with an aspartate at P4 along with the backbone nitrogen of F250, creating a preference for a carboxylic acid at the P4 site. |
Revision as of 00:48, 13 December 2012
Introduction
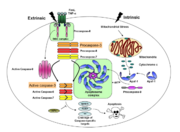
Caspases are cysteine-aspartic acid proteases and are key protein facilitators for the faithful execution of apoptosis or programmed cell death. Dysregulation in the apoptotic pathway has been implicated in a variety of diseases such as neurodegeneration, cancer, heart disease and some metabolic disorders. Because of the crucial role of caspases in the the apoptotic pathway, abnormalities in their functions would cause a haywire in the apoptotic cascade and can be deleterious to the cell. Caspases are thus being considered as therapeutic targets in apoptosis-related diseases.
Any apoptotic signal received by the cell causes the activation of initiator caspases (-8 and -9) by associating with other protein platforms to form a functional holoenzyme. These initiator caspases then cleaves the executioner caspases -3, -6, -7. Caspase-3 specifically functions to cleave both caspase-6 and -7, which in turn cleave their respective targets to induce cell death. Aside from being able to activate caspase-6 and -7, caspase-3 also regulates caspase-9 activity, operating via a feedback loop. These dual action of caspase-3 confers its distinct regulatory mechanisms, resulting a wider extent of its effects in the apoptotic cascade.
| |||||||||||
Caspase-3 Loop Bundle and Active Site
| |||||||||||
Caspase-3 Regulation
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Scott Eron, Banyuhay P. Serrano, Alexander Berchansky, Yunlong Zhao, Jaime Prilusky, Michal Harel