This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox UC 10
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
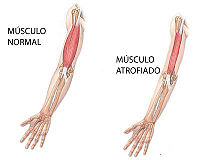
CONCLUSIONS: The dystrophin ABD structure reveals a previously uncharacterised arrangement of the CH domains within the ABD. This observation has implications for the mechanism of actin binding by dystrophin and related proteins. Examining the position of three pathogenic missense mutations within the structure suggests that they exert their effects through misfolding of the ABD, rather than through disruption of the binding to F-actin<ref>PMID 10801490</ref>[[Image:imag1gonzalo.jpg|right|200px|thumb|Comparacion de la degeneracion muscular, [[1dxx]]]] | CONCLUSIONS: The dystrophin ABD structure reveals a previously uncharacterised arrangement of the CH domains within the ABD. This observation has implications for the mechanism of actin binding by dystrophin and related proteins. Examining the position of three pathogenic missense mutations within the structure suggests that they exert their effects through misfolding of the ABD, rather than through disruption of the binding to F-actin<ref>PMID 10801490</ref>[[Image:imag1gonzalo.jpg|right|200px|thumb|Comparacion de la degeneracion muscular, [[1dxx]]]] | ||
| - | |||
| - | [[Image:MW_Folding_Simulations.gif|200px|thumb|[[1dxx]]]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
== Escenas == | == Escenas == | ||
| - | <scene name=' | + | <scene name='Sandbox_UC_10/Conservacion_evolutiva/1'>Zonas conservadas evolutivamente</scene> |
{{Template:ColorKey_Amino2CarboxyRainbow}} | {{Template:ColorKey_Amino2CarboxyRainbow}} | ||
Revision as of 20:25, 19 December 2012
N-TERMINAL ACTIN-BINDING DOMAIN OF HUMAN DYSTROPHIN
| |||||||||||
QUIZ TIME !!
Referencias
- ↑ Norwood FL, Sutherland-Smith AJ, Keep NH, Kendrick-Jones J. The structure of the N-terminal actin-binding domain of human dystrophin and how mutations in this domain may cause Duchenne or Becker muscular dystrophy. Structure. 2000 May 15;8(5):481-91. PMID:10801490
- ↑ Nadeau D, Marchand C. Change in the kinetics of sulphacetamide tissue distribution in Walker tumor-bearing rats. Drug Metab Dispos. 1975 Nov-Dec;3(6):565-76. PMID:1234
- ↑ Rubinstein MH. A new granulation method for compressed tablets [proceedings]. J Pharm Pharmacol. 1976 Dec;28 Suppl:67P. PMID:12345