We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 714
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
=== C-terminal domain === | === C-terminal domain === | ||
| - | The C-terminal domain is called Cytosolic epoxide hydrolase 2: it catalyzes the trans-addition of water to epoxides in order to product glycols<ref>PMID:15822179</ref>. The <scene name='Sandbox_Reserved_714/Cter_activesite/3'>active site</scene> is made of five residues. The 3D structure of this active site is maintained by hydrogen bonds, including those created by D496. The two tyrosines (Y383 and Y466) assist the proper positioning of the substrate by polarizing it, thanks to their hydroxyl groups. D335 acts as a nucleophilic acid. Finally, H524 plays the role of a base in order to release the final product. | + | The C-terminal domain is called Cytosolic epoxide hydrolase 2: it catalyzes the trans-addition of water to epoxides in order to product glycols<ref>PMID:15822179</ref>. |
| + | |||
| + | The corresponding reaction equation is the following: | ||
| + | Epoxide + H<sub>2</sub>O ↔ Glycol | ||
| + | |||
| + | The <scene name='Sandbox_Reserved_714/Cter_activesite/3'>active site</scene> is made of five residues. The 3D structure of this active site is maintained by hydrogen bonds, including those created by D496. The two tyrosines (Y383 and Y466) assist the proper positioning of the substrate by polarizing it, thanks to their hydroxyl groups. D335 acts as a nucleophilic acid. Finally, H524 plays the role of a base in order to release the final product. | ||
The reaction proceeds in two steps, including the formation of a covalent intermediate. | The reaction proceeds in two steps, including the formation of a covalent intermediate. | ||
First, the substrate (epoxide) is accepted in the active site and its oxygen forms hydrogen bonds with Y383 and Y466. The oxygen of D335 attacks one of the two carbons included in the epoxide function. As a result, the oxygen of the subtrate takes the hydrogen of the hydroxyl function of Y466: the covalent intermediate is formed, and linked to D335. | First, the substrate (epoxide) is accepted in the active site and its oxygen forms hydrogen bonds with Y383 and Y466. The oxygen of D335 attacks one of the two carbons included in the epoxide function. As a result, the oxygen of the subtrate takes the hydrogen of the hydroxyl function of Y466: the covalent intermediate is formed, and linked to D335. | ||
Then, the oxygen negatively charged belonging to the lateral chain of Y383 attacks the hydrogen of H524. After that, a water molecule enters the active site. The hydroxyl of D335 is therefore renewed, the diol product is created and released. The active site is available for a new catalytic cycle. | Then, the oxygen negatively charged belonging to the lateral chain of Y383 attacks the hydrogen of H524. After that, a water molecule enters the active site. The hydroxyl of D335 is therefore renewed, the diol product is created and released. The active site is available for a new catalytic cycle. | ||
| - | |||
| - | The corresponding reaction equation is the following: | ||
| - | |||
| - | Epoxide + H<sub>2</sub>O ↔ Glycol | ||
=== N-terminal domain === | === N-terminal domain === | ||
Revision as of 15:44, 2 January 2013
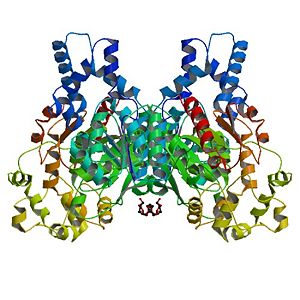
Human Soluble Epoxide Hydrolase: Biological assembly, 1s8o
Contents |
Overview
| |||||||||||
External ressources
References
- ↑ Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311-33. PMID:15822179 doi:10.1146/annurev.pharmtox.45.120403.095920
- ↑ Gomez GA, Morisseau C, Hammock BD, Christianson DW. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry. 2004 Apr 27;43(16):4716-23. PMID:15096040 doi:10.1021/bi036189j
Proteopedia Page Contributors and Editors
DUTREUX Fabien, BONHOURE Anna

