This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 714
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
The N-terminal domain is responsible of the Mg<sup>2+</sup> dependant hydrolysis of dihydroxy lipid phosphates <ref>PMID:15096040</ref>. Indeed, the aliphatic substrate binds the protein on its hydrophobic tunnel, as it has been described previously. The specificity of this enzyme has been tested for several lipid molecules, and the best substrate found is the monophosphate of dihydroxy stearic acid (threo-9/10-phosphonoxy-hydroxy-octadecanoic acid) <ref>PMID:12574510</ref>. | The N-terminal domain is responsible of the Mg<sup>2+</sup> dependant hydrolysis of dihydroxy lipid phosphates <ref>PMID:15096040</ref>. Indeed, the aliphatic substrate binds the protein on its hydrophobic tunnel, as it has been described previously. The specificity of this enzyme has been tested for several lipid molecules, and the best substrate found is the monophosphate of dihydroxy stearic acid (threo-9/10-phosphonoxy-hydroxy-octadecanoic acid) <ref>PMID:12574510</ref>. | ||
| + | |||
| + | In the example of this substrate, the reaction follows this equation: | ||
| + | 9/10-phosphonoxy-hydroxy-octadecanoate + H<sub>2</sub>O ↔ 9/10-dihydroxy-octadecanoate + phosphate | ||
Its <scene name='Sandbox_Reserved_714/Nter_activesite/1'>active site</scene> contains several conserved aspartates in phosphatases and phosphonatases: D9, D11, D184 and D185. This enzymatic activity is Mg<sup>2+</sup> dependant, because the structure of the active site is in its optimal conformation when the cation makes coordination interactions. When the catalytic activity of the N-term domain is available, Magnesium is octahedrally coordinated with the four aspartates, one water molecule and the phosphate belonging to the substrate. | Its <scene name='Sandbox_Reserved_714/Nter_activesite/1'>active site</scene> contains several conserved aspartates in phosphatases and phosphonatases: D9, D11, D184 and D185. This enzymatic activity is Mg<sup>2+</sup> dependant, because the structure of the active site is in its optimal conformation when the cation makes coordination interactions. When the catalytic activity of the N-term domain is available, Magnesium is octahedrally coordinated with the four aspartates, one water molecule and the phosphate belonging to the substrate. | ||
Revision as of 16:33, 2 January 2013
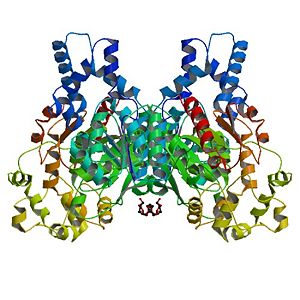
Human Soluble Epoxide Hydrolase: Biological assembly, 1s8o
Contents |
Overview
| |||||||||||
External ressources
References
- ↑ Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311-33. PMID:15822179 doi:10.1146/annurev.pharmtox.45.120403.095920
- ↑ Gomez GA, Morisseau C, Hammock BD, Christianson DW. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry. 2004 Apr 27;43(16):4716-23. PMID:15096040 doi:10.1021/bi036189j
- ↑ Newman JW, Morisseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1558-63. Epub 2003 Feb 6. PMID:12574510 doi:10.1073/pnas.0437724100
Proteopedia Page Contributors and Editors
DUTREUX Fabien, BONHOURE Anna

