Image:CuI coordination.png
From Proteopedia
(Difference between revisions)
No higher resolution available.
CuI_coordination.png (614 × 460 pixel, file size: 162 KB, MIME type: image/png)
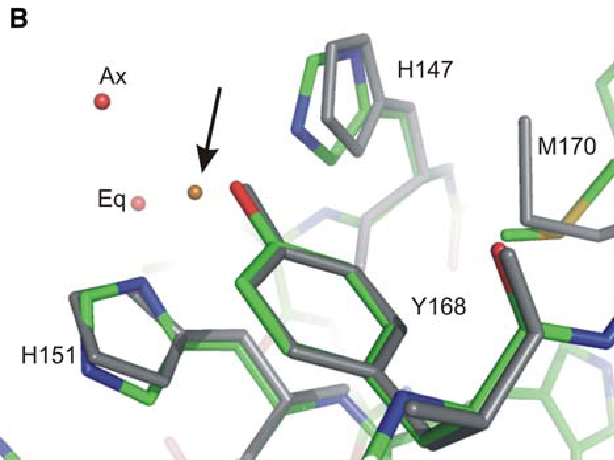
(Our structure derived from such crystals revealed that the two histidines (His147, His151) and the tyrosine residue (Tyr168) participate in Cu(II) binding but not the methionine residue. In addition, two water ligands were identified: an axial water and a) |
(uploaded a new version of "Image:CuI coordination.png": This structure was obtain by X-ray fluorescence scan this structure revealed that the two histidines (His147, His151) and the tyrosine residue (Tyr168) participate in Cu(II) binding but not the m) |
Revision as of 16:13, 4 January 2013
Summary
Our structure derived from such crystals revealed that the two histidines (His147, His151) and the tyrosine residue (Tyr168) participate in Cu(II) binding but not the methionine residue. In addition, two water ligands were identified: an axial water and an ‘equatorial’ water molecule located ∼1.5 Å above the plane of the other ligands
Licensing
{{subst:No license from license selector|Don't know}}
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 16:13, 4 January 2013 | Andréa Mc Cann (Talk | contribs) | 614×460 | 162 KB | This structure was obtain by X-ray fluorescence scan this structure revealed that the two histidines (His147, His151) and the tyrosine residue (Tyr168) participate in Cu(II) binding but not the methionine residue. In addition, two water ligands were ident |
| 15:58, 4 January 2013 | Andréa Mc Cann (Talk | contribs) | 614×460 | 162 KB | Our structure derived from such crystals revealed that the two histidines (His147, His151) and the tyrosine residue (Tyr168) participate in Cu(II) binding but not the methionine residue. In addition, two water ligands were identified: an axial water and a |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
