Sandbox Reserved 707
From Proteopedia
| Line 14: | Line 14: | ||
The B-RAF Protein belongs to a large family of protein kinases that have a very important role on different aspects of cellular biology and biomedical sciences. The human protein kinase family consists of 518 genes, making it one of the largest gene families. Based upon the phosphorylation of the hydroxyl group these proteins are classified on 3 groups: protein serine threonine kinase (385 members), protein tyrosine kinase (90 members) and tyrosine kinase like proteins (43 members). A-RAF, B-RAF and C-RAF proteins are a family of serine threonine kinase proteins that participate in the RAS-RAF-MEK-ERK signal transduction cascade. The general reaction they catalyse is: | The B-RAF Protein belongs to a large family of protein kinases that have a very important role on different aspects of cellular biology and biomedical sciences. The human protein kinase family consists of 518 genes, making it one of the largest gene families. Based upon the phosphorylation of the hydroxyl group these proteins are classified on 3 groups: protein serine threonine kinase (385 members), protein tyrosine kinase (90 members) and tyrosine kinase like proteins (43 members). A-RAF, B-RAF and C-RAF proteins are a family of serine threonine kinase proteins that participate in the RAS-RAF-MEK-ERK signal transduction cascade. The general reaction they catalyse is: | ||
| - | == Structure<ref>PMID: 15035987</ref> == | + | |
| + | |||
| + | This cascade participates in the regulation of several cellular mechanisms like: apoptosis, cell cycle progression, differentiation, proliferation and transformation to the cancerous state in response to growth factors, cytokines and hormones. First discovered in 1938 as a retroviral oncogene B-RAF plays a key role on all studies concerning cancer therapies and other biomedical applications. | ||
| + | |||
| + | == B-RAF Structure<ref>PMID: 15035987</ref> == | ||
<Structure load='1UWH' size='400' frame='true' align='left' caption='3D View of the complex' scene='Insert optional scene name here' /> | <Structure load='1UWH' size='400' frame='true' align='left' caption='3D View of the complex' scene='Insert optional scene name here' /> | ||
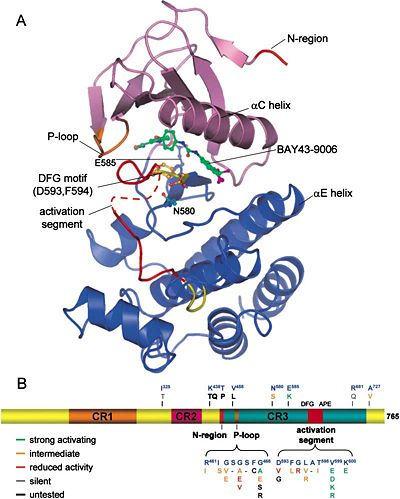
[[Image:1-s2.0-S0092867404002156-main page7 image1.jpg|400px|thumb| A. Structure of B-RAF Kinase Domain <br /> | [[Image:1-s2.0-S0092867404002156-main page7 image1.jpg|400px|thumb| A. Structure of B-RAF Kinase Domain <br /> | ||
B. Schematic B-RAF Primary Structure]] | B. Schematic B-RAF Primary Structure]] | ||
| - | + | ||
| + | As we distinguished before, there are 3 types of RAF proteins: A-RAF, B-RAF and C-RAF. All of them share 3 much conserved regions (CR): CR1, CR2 and CR3. | ||
| + | CR1 is composed of a RAS binding domain (RBD) and a cysteine rich domain (CRD) which can bind 2 zinc ions (zinc finger domain). It is possible for the CR1 to interact with the RAS and with the membrane phospholipids (PH domain) | ||
| + | CR2 is a serine threonine rich domain. When a serine is phosphorylated this domain can bind a regulatory protein that can bind the C-terminal region in the same time. Binding of 14-3-3 (C-terminal region) to this phosphorylated serine is inhibitory for the enzyme. | ||
| + | CR3 is the protein kinase domain on the C-terminal region. We can find just after this kinase domain a stimulatory 14-3-3 binding site for other regulatory proteins. | ||
| + | |||
== Regulation == | == Regulation == | ||
Revision as of 22:36, 4 January 2013
The complex of wild type B-RAF and BAY439006
Contents |
Introduction
The B-RAF Protein belongs to a large family of protein kinases that have a very important role on different aspects of cellular biology and biomedical sciences. The human protein kinase family consists of 518 genes, making it one of the largest gene families. Based upon the phosphorylation of the hydroxyl group these proteins are classified on 3 groups: protein serine threonine kinase (385 members), protein tyrosine kinase (90 members) and tyrosine kinase like proteins (43 members). A-RAF, B-RAF and C-RAF proteins are a family of serine threonine kinase proteins that participate in the RAS-RAF-MEK-ERK signal transduction cascade. The general reaction they catalyse is:
This cascade participates in the regulation of several cellular mechanisms like: apoptosis, cell cycle progression, differentiation, proliferation and transformation to the cancerous state in response to growth factors, cytokines and hormones. First discovered in 1938 as a retroviral oncogene B-RAF plays a key role on all studies concerning cancer therapies and other biomedical applications.
B-RAF Structure[1]
|
As we distinguished before, there are 3 types of RAF proteins: A-RAF, B-RAF and C-RAF. All of them share 3 much conserved regions (CR): CR1, CR2 and CR3. CR1 is composed of a RAS binding domain (RBD) and a cysteine rich domain (CRD) which can bind 2 zinc ions (zinc finger domain). It is possible for the CR1 to interact with the RAS and with the membrane phospholipids (PH domain) CR2 is a serine threonine rich domain. When a serine is phosphorylated this domain can bind a regulatory protein that can bind the C-terminal region in the same time. Binding of 14-3-3 (C-terminal region) to this phosphorylated serine is inhibitory for the enzyme. CR3 is the protein kinase domain on the C-terminal region. We can find just after this kinase domain a stimulatory 14-3-3 binding site for other regulatory proteins.
Regulation
signaling ways
Other Inhibitors
drugs and inhibitor paradox
Applications in Medicine
External Ressources
References
- ↑ Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004 Mar 19;116(6):855-67. PMID:15035987
Protreopedia Page Contributors and Editors
Kostika Sofroni and Gilles Dupouy