Sandbox Reserved 716
From Proteopedia
m (→'''3D structure''') |
m |
||
| Line 33: | Line 33: | ||
[[Image:Selectin_Structure.png|800px|center]] | [[Image:Selectin_Structure.png|800px|center]] | ||
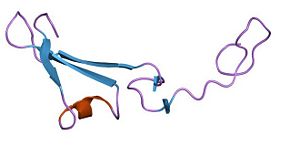
| - | [[Image:PDB 1hre EBI2.jpg|300px|right|thumb| EGF domain]] EGF domain (Epidermal Growth Factor) coutains 30 to 40 amino-acids residues. This domain is composed by 6 cysteine residues which can form 3 disulfide bonds. EGF domain is formed with two-stranded β-sheet followed by a loop to a short C-terminal two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. | + | [[Image:PDB 1hre EBI2.jpg|300px|right|thumb| EGF domain]] EGF domain (Epidermal Growth Factor) coutains 30 to 40 amino-acids residues. This domain is composed by 6 cysteine residues which can form 3 disulfide bonds. EGF domain is formed with two-stranded β-sheet followed by a loop to a short C-terminal two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. |
EGF domain seems to be useful for three kind of interactions : | EGF domain seems to be useful for three kind of interactions : | ||
| Line 42: | Line 42: | ||
- '''protein-protein interactions''' | - '''protein-protein interactions''' | ||
| - | |||
| Line 49: | Line 48: | ||
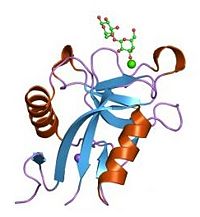
[[Image:Lectin domain.jpg|200px|left|thumb| Lectin domain bound to sugar]] | [[Image:Lectin domain.jpg|200px|left|thumb| Lectin domain bound to sugar]] | ||
| - | |||
| - | |||
Lectin domains (also known as C-type lectin domains) are classified in 17 groups (from I to XVII). P-Selectin lectin bellow to the group IV. | Lectin domains (also known as C-type lectin domains) are classified in 17 groups (from I to XVII). P-Selectin lectin bellow to the group IV. | ||
The P-selectin lectin domain binds (via an extended site of the domain) a ligand : '''the carbohydrate sialyl LewisX (SLeX)''' with low affinity. However, the affinity between the ligand and the selectins is actually quite high. A theory may explain why lectin domain may recognize SLeX (or related carbohydrate structures on this ligand) with a higher affinity. Indeed EGF domain may alter the conformation of the lectin domain so that its affinity for the ligand is increased. | The P-selectin lectin domain binds (via an extended site of the domain) a ligand : '''the carbohydrate sialyl LewisX (SLeX)''' with low affinity. However, the affinity between the ligand and the selectins is actually quite high. A theory may explain why lectin domain may recognize SLeX (or related carbohydrate structures on this ligand) with a higher affinity. Indeed EGF domain may alter the conformation of the lectin domain so that its affinity for the ligand is increased. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
== '''Different role of the P-selectin''' == | == '''Different role of the P-selectin''' == | ||
| Line 72: | Line 82: | ||
== '''References''' == | == '''References''' == | ||
| + | |||
1. http://cro.sagepub.com/content/10/3/337.full.pdf | 1. http://cro.sagepub.com/content/10/3/337.full.pdf | ||
| Line 79: | Line 90: | ||
4. Phan UT, Waldron TT, Springer TA (2006). "Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force". Nat Immunol 7 (8): 883–9. doi:10.1038/ni1366. PMID 16845394. | 4. Phan UT, Waldron TT, Springer TA (2006). "Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force". Nat Immunol 7 (8): 883–9. doi:10.1038/ni1366. PMID 16845394. | ||
| + | |||
== '''Proteopedia Page Contributors and Editors''' == | == '''Proteopedia Page Contributors and Editors''' == | ||
Delphine Trelat, Cécile Ehrhardt | Delphine Trelat, Cécile Ehrhardt | ||
Revision as of 16:30, 5 January 2013
|
Crystal structure of P-selectin lectin/EGF domains complexed with SLeX
Contents |
Introduction
Selectins are proteins that are include in a family of cell adhesion receptor involved in the leukocyte extravasation. There are 3 kinds of selectins :
E selectin localized in endothelial cells, L selectin found in leukocytes, and P selectins in platelets and endothelial cells.
In this page we will be focused only on P-Selectin.
When the P-selectin is activated by molecules like histamine or thrombin, it is able to bind myeloid cells and some T cells.
3D structure
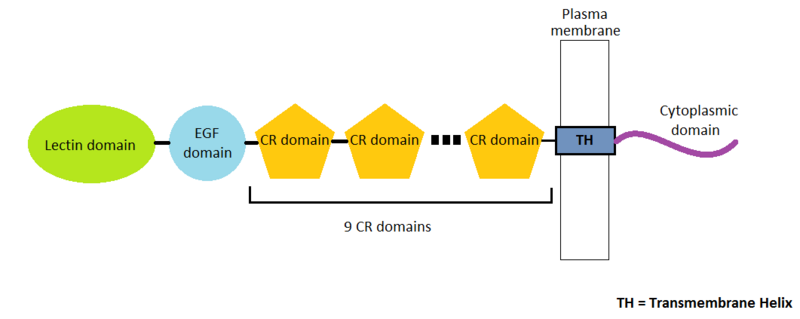
P-Selectin is a protein composed by 162 amino-acids residues in 4 different chains A, B, C and D. There are many domains in this protein. Three types of domains represent the extracellular part of the protein : the EGF domain, the lectin domain and nine consensus repeat (CR) domains. Finally, we find a transmembrane helix and a cytoplasmic domain.
In this page we will only detail the EGF domain and the lectin domain.
EGF domain (Epidermal Growth Factor) coutains 30 to 40 amino-acids residues. This domain is composed by 6 cysteine residues which can form 3 disulfide bonds. EGF domain is formed with two-stranded β-sheet followed by a loop to a short C-terminal two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets.EGF domain seems to be useful for three kind of interactions :
- ligand recognition : The main ligand for P-selectin is P-selectin glycoprotein ligand-1 (PSGL-1)
- cell adhesion : adhesion of the leukocyte to the vessel membrane
- protein-protein interactions
Lectin domains (also known as C-type lectin domains) are classified in 17 groups (from I to XVII). P-Selectin lectin bellow to the group IV.
The P-selectin lectin domain binds (via an extended site of the domain) a ligand : the carbohydrate sialyl LewisX (SLeX) with low affinity. However, the affinity between the ligand and the selectins is actually quite high. A theory may explain why lectin domain may recognize SLeX (or related carbohydrate structures on this ligand) with a higher affinity. Indeed EGF domain may alter the conformation of the lectin domain so that its affinity for the ligand is increased.
Different role of the P-selectin
Role in leukocyte extravasation
Leukocyte extravasation is the movement of leukocytes out of the circulatory system at the time of an infection. The inflammation is a natural reaction of defense caused by tissular damages. The recruitement of leukocytes on the inflammation site is a process in four steps. First, the leukocyte is attracted by cytokines, secreted near the site of infection. This is the chemoattraction. Then, this leukocyte begin rolling along the inner surface of the vessel, binding on selectin molecules. The third step is the tight adhesion, the leukocyte immobilizates himself, thanks to the integrin molecules. And finally, he passes through gaps between endothelial cells. By this mecanism, the leukocyte arrives on the site of infection to neutralize the infection agent.
Role in platelets recruitment
Role in cancer
External resources
Protein Data Bank file 1G1R
References
1. http://cro.sagepub.com/content/10/3/337.full.pdf
2. Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000 Oct 27;103(3):467-79. PMID 11081633.
3. Kansas GS, Saunders KB, Ley K, et al (1994). "A role for the epidermal growth factor-like domain of P-selectin in ligand recognition and cell adhesion". J Cell Biol 124 (4): 609–18. PMID 7508943.
4. Phan UT, Waldron TT, Springer TA (2006). "Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force". Nat Immunol 7 (8): 883–9. doi:10.1038/ni1366. PMID 16845394.
Proteopedia Page Contributors and Editors
Delphine Trelat, Cécile Ehrhardt