User:Charlotte Kern/Sandbox 702
From Proteopedia
| Line 18: | Line 18: | ||
====Protective antigen==== | ====Protective antigen==== | ||
| - | PA binds mammalian receptors CMG2 (capillary morphogenesis gene 2) and TEM8 (tumor endothelial marker 8). PA promotes EF and LF translocation in the cytosol of infected cells, particularly macrophages, where the two enzymes perform their damage-inducing processes, allowing bacteria to evade the immune system. | + | PA binds mammalian receptors CMG2 (capillary morphogenesis gene 2) and TEM8 (tumor endothelial marker 8). PA promotes EF and LF translocation in the cytosol of infected cells, particularly macrophages, where the two enzymes perform their damage-inducing processes, allowing bacteria to evade the immune system. <ref>Tournier, J.N.; Rossi Paccani, S.; Quesnel-Hellmann, A.; Baldari, C.T. Anthrax toxins: A weapon to systematically dismantle the host immune defenses. Mol. Aspects Med. 2009, 30, 456–466</ref> |
====Lethal factor==== | ====Lethal factor==== | ||
| - | LF is a zinc-mediated metalloprotease that cleaves mitogen-activated protein kinase kinases (MEKs). This impairs cell signaling, and results in the induction of apoptosis. | + | LF is a zinc-mediated metalloprotease that cleaves mitogen-activated protein kinase kinases (MEKs). This impairs cell signaling, and results in the induction of apoptosis. <ref>Klimpel, K. R., N. Arora, and S. H. Leppla. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 13:1093–1100</ref> |
====Edema factor==== | ====Edema factor==== | ||
| - | EF is a calmodulin-dependent adenylyl cyclase that depletes cellular adenosine triphosphate (ATP) while creating 3',5'-cyclic adenosine monophosphate (cAMP), a cellular second messenger. | + | EF is a calmodulin-dependent adenylyl cyclase that depletes cellular adenosine triphosphate (ATP) while creating 3',5'-cyclic adenosine monophosphate (cAMP), a cellular second messenger. <ref>Leppla, 1982, Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cAMP concentration in eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3163</ref> |
Both toxins can be reconstituted vitro, by combining PA with LF or EF, to form lethal toxin (LT) or edema toxin (ET). | Both toxins can be reconstituted vitro, by combining PA with LF or EF, to form lethal toxin (LT) or edema toxin (ET). | ||
Revision as of 23:41, 5 January 2013
| |||||||||
| 1lvc, resolution 3.60Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Activity: | Adenylate cyclase, with EC number 4.6.1.1 | ||||||||
| Related: | 1k90 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
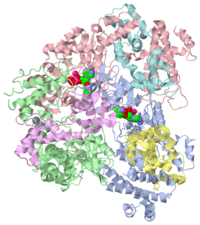
Anthrax edema factor (EF) is an enzyme which is part of the Bacillus anthracis anthrax toxin. Here we study 1lvc – EF adenylate cyclase domain + calmodulin + anthraniloyl-deoxy-ATP.
The edema factor is a calmodulin-dependent adenylate cyclase. Adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] increases intracellular cyclic AMP (cAMP) concentrations in eukaryotic cells. In fact adenylyl cyclases catalyze the conversion of adenosine triphosphate (ATP) into cAMP and pyrophosphate. The cellular level of cAMP increases, upsetting water homeostasis and causing disruption of signaling pathways.
EF is produced in an inactive form. When it is in the cell, EF adenylyl cyclase activity is induced by complexation with calmodulin, so it is allosterically activated. Its enzymatic activity leads to a dramatic elevation of the cAMP range. Calmodulin is ubiquitous eukaryotic cellular protein and a Ca2+ ion sensor present in host cells. Cyclic AMP is a second messenger that plays key roles in the signal transduction pathways and thus regulates diverse cellular responses. It binds to three families of signal transducers: cAMP-dependent protein kinases, cyclic nucleotide gated channels, and the guanine nucleotide exchange factor for Ras GTPase homologs Rap1 and Rap2 (EPAC). [1] [2]
Contents |
Anthrax toxin
The anthrax toxin is composed of a cell-binding protein (protective antigen) (PA)(83kDa), lethal factor (LF)(90kDa) and edema factor (EF)(89kDa). Edema factor, protective antigen and lethal factor can also be called factor I, II and III respectively. [3] [4]
The complex produced by Bacillus anthracis consists of a virulent mixture of two toxins, with different enzymatic activities. They have a similar N-terminal domain that allows them to bind to a large protein: Protective Antigen.
Protective antigen
PA binds mammalian receptors CMG2 (capillary morphogenesis gene 2) and TEM8 (tumor endothelial marker 8). PA promotes EF and LF translocation in the cytosol of infected cells, particularly macrophages, where the two enzymes perform their damage-inducing processes, allowing bacteria to evade the immune system. [5]
Lethal factor
LF is a zinc-mediated metalloprotease that cleaves mitogen-activated protein kinase kinases (MEKs). This impairs cell signaling, and results in the induction of apoptosis. [6]
Edema factor
EF is a calmodulin-dependent adenylyl cyclase that depletes cellular adenosine triphosphate (ATP) while creating 3',5'-cyclic adenosine monophosphate (cAMP), a cellular second messenger. [7] Both toxins can be reconstituted vitro, by combining PA with LF or EF, to form lethal toxin (LT) or edema toxin (ET).
Edema toxin (ET)
ET is the combination of PA and EF, inducing pathogenic effects. PA is the dominant antigen for immunization, that’s why it is more studied for therapeutic efforts, but LF and EF are also targeted as they are important effectors during anthrax infection.
Crystal structure of the adenylyl cyclase domain of anthrax edema factor (EF) in complex with calmodulin and 2' deoxy, 3' anthraniloyl ATP
Edema factor (EF) and CyaA are calmodulin (CaM)-activated adenylyl cyclase exotoxins involved in the pathogenesis of anthrax and whooping cough, respectively. Using spectroscopic, enzyme kinetic and surface plasmon resonance spectroscopy analyses, we show that low Ca(2+) concentrations increase the affinity of CaM for EF and CyaA causing their activation, but higher Ca(2+) concentrations directly inhibit catalysis. Both events occur in a physiologically relevant range of Ca(2+) concentrations. Despite the similarity in Ca(2+) sensitivity, EF and CyaA have substantial differences in CaM binding and activation. CyaA has 100-fold higher affinity for CaM than EF. CaM has N- and C-terminal globular domains, each binding two Ca(2+) ions. CyaA can be fully activated by CaM mutants with one defective C-terminal Ca(2+)-binding site or by either terminal domain of CaM while EF cannot. EF consists of a catalytic core and a helical domain, and both are required for CaM activation of EF. Mutations that decrease the interaction of the helical domain with the catalytic core create an enzyme with higher sensitivity to Ca(2+)-CaM activation. However, CyaA is fully activated by CaM without the domain corresponding to the helical domain of EF.
Physiological calcium concentrations regulate calmodulin binding and catalysis of adenylyl cyclase exotoxins., Shen Y, Lee YS, Soelaiman S, Bergson P, Lu D, Chen A, Beckingham K, Grabarek Z, Mrksich M, Tang WJ, EMBO J. 2002 Dec 16;21(24):6721-32. PMID:12485993
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
1lvc is a 6 chain structure with sequence from Bacillus anthracis and Homo sapiens. Full crystallographic information is available from OCA.
See Also
Reference
- ↑ Fouet, A. 2009. The surface of Bacillus anthracis. Mol. Aspects Med. 30:374–385
- ↑ Moayeri, M., and S. H. Leppla. 2009. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 30:439–455
- ↑ Fouet, A. 2009. The surface of Bacillus anthracis. Mol. Aspects Med. 30:374–385
- ↑ Moayeri, M., and S. H. Leppla. 2009. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 30:439–455
- ↑ Tournier, J.N.; Rossi Paccani, S.; Quesnel-Hellmann, A.; Baldari, C.T. Anthrax toxins: A weapon to systematically dismantle the host immune defenses. Mol. Aspects Med. 2009, 30, 456–466
- ↑ Klimpel, K. R., N. Arora, and S. H. Leppla. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 13:1093–1100
- ↑ Leppla, 1982, Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cAMP concentration in eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3163