User:Charlotte Kern/Sandbox 702
From Proteopedia
| Line 56: | Line 56: | ||
[[A REFORMULER : | [[A REFORMULER : | ||
| - | The surface-exposed residues in L5, a6, a7, and the joining loop between a7 and a8 at the C-terminal five-helix domain of LF-PABD and EF-PABD have been mapped by mutational analysis to make the primary contacts with PA (Lacy et al, 2002). The PA binding surface of EF–PABD is richly negatively charged, which could promote interaction with the positively charged residues at EF/LF interacting surface of PA (Cunningham et al, 2002) | + | The surface-exposed residues in L5, a6, a7, and the joining loop between a7 and a8 at the C-terminal five-helix domain of LF-PABD and EF-PABD have been mapped by mutational analysis to make the primary contacts with PA (Lacy et al, 2002). The PA binding surface of EF–PABD is richly negatively charged, which could promote interaction with the positively charged residues at EF/LF interacting surface of PA (Cunningham et al, 2002)]] |
==The molecular basis of activation and catalysis of the adenylyl cyclase activity of EF by calmodulin== | ==The molecular basis of activation and catalysis of the adenylyl cyclase activity of EF by calmodulin== | ||
| Line 64: | Line 64: | ||
==The molecular basis for the activation of anthrax EF by calmodulin== | ==The molecular basis for the activation of anthrax EF by calmodulin== | ||
| + | [[........ j'ai pas continué à mettre ta partie, mais je me suis occupée de la mienne :)]] | ||
| + | |||
| + | ==Prevention and treatment== | ||
| + | It is possible to use classical therapeutic approaches to fight anthrax disease. Since the original vaccine trials by Louis Pasteur in 1881, an attenuated Stern strain is still successfully used as a vaccine in livestock. Many human vaccines based on PA have been developed in the 1960s. | ||
| + | |||
| + | A large number of treatments against anthrax toxin currently in development are antibodies. The majority of these antibodies target the receptor binding domain of PA, blocking binding of PA to cellular receptors. <ref>Young, J. A., and R. J. Collier. 2007. Anthrax toxin: receptor-binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76:243–265 </ref> | ||
| + | |||
| + | Efforts to make antibodies to EF have had varied levels of success. | ||
| + | Immunoglobulin G (IgG) have been produced, antibodies of moderate affinity to EF, one of which (9F5) was able to inhibit binding of EF to PA and prevent physiological effects of EF on Chinese hamster ovary (CHO) cells. <ref> Little, S. F., S. H. Leppla, J. W. Burnett, and A. M. Friedlander. 1994. Structure-function analysis of Bacillus anthracis edema factor by using monoclonal antibodies. Biochem. Biophys. Res. Commun. 199:676–682</ref> | ||
| + | |||
| + | Winterroth and colleagues described six antibodies of moderate affinity, including one IgM that could neutralize EF activity in CHO cells. This antibody had no significant protective effect against a Sterne strain infection in a mouse model but extended the mean time to death when combined with a subprotective dose of anti-PA antibody. <ref>Winterroth, L., J. Rivera, A. S. Nakouzi, E. Dadachova, and A. Casadevall. 2010. A neutralizing monoclonal antibody to edema toxin and its effect on murine anthrax. Infect. Immun. 78:2890–2896 </ref> | ||
| + | |||
| + | Chen and colleagues developed chimpanzee antibodies that included one having very high affinity and that competes with calmodulin for binding to the helical domain of EF. This antibody was very effective at preventing ET-mediated edema in a mouse footpad model and provided significant protection in a systemic toxin challenge. <ref>Chen, Z., et al. 2009. Potent neutralization of anthrax edema toxin by a humanized monoclonal antibody that competes with calmodulin for edema factor binding. Proc. Natl. Acad. Sci. U. S. A. 106:13487–13492</ref> | ||
| + | |||
| + | Leysath et al. developed and characterized four anti-EF monoclonal antibodies (MAb). | ||
| + | MAb bind to epitopes on three different domains of EF: PA binding domain, catalytic CB domain, and helical domain. They showed that three of the four IgGs have neutralizing activity in vitro and in vivo, inhibiting production of cAMP. Antibody 7F10 has the highest efficacy and 3F2 is the first monoclonal antibody to EF whose epitope is on the catalytic CB domain. | ||
| + | MAb are highly specific for EF and do not cross-react with LF or PA and can decrease edema levels and progression of disease in mouse models. | ||
| + | These antibodies can serve as reagents in diagnostics assays, and can be useful reagents for further molecular studies of EF action. <ref>Leysath, C.E. et al., Mouse Monoclonal Antibodies to Anthrax Edema Factor Protect against Infection. Infection and immunity, Nov. 2011, p. 4609–4616</ref> | ||
| + | |||
| + | ====Inhibitors==== | ||
| + | Schein et al. identified non-nucleotide inhibitors of EF. | ||
| + | Inhibitors targeting sites for such allosteric activators have recently been identified. <ref>Laine, É.; Martínez, L.; Ladant, D.; Malliavin, T.; Blondel, A. Molecular motions as a drug target: Mechanistic simulations of anthrax toxin edema factor function led to the discovery of novel allosteric inhibitors. Toxins 2012, 4, 580–604</ref> | ||
| + | They chose to do a direct design based on analysis of the structure of the substrate binding site of the EF protein, rather than a design starting from modifying nucleotides related to the substrate itself. Their studies focused on the active site (circled in the structure of EF bound to calmodulin, shown in Figure 1Top). Comparison of the active site conformation in various crystal structures in the Protein database (PDB) (which differed in the number and types of bound metal ions and substrates [16]) revealed important information about how the active site of the toxin differed from the mammalian adenyl cyclase enzymes. These crystal structures, with or without the bound metal ions, were used for docking potential inhibitors identified by our fragment based pharmacophore. | ||
| + | Our previous studies [16] indicated that the docking of 3'-dATP to the crystal structure of EF (PDB structure: 1K90 <ref>Drum, C.L.; Yan, S.Z.; Bard, J.; Shen, Y.Q.; Lu, D.; Soelaiman, S.; Grabarek, Z.; Bohm, A.; Tang, W.J. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature 2002, 415, 396–402</ref> had low RMSD (root-mean-square deviations) between the predicted structure and the crystal structure, and thus, AutoDock 3.0 was reliable enough to predict the binding mode of the ligands to EF. | ||
| + | Although early treatment with antibiotics can greatly reduce the effects of bacterial infections, inhibitors of the toxins could play a therapeutic role in later stage infections, in preventing toxin induced diarrhea or even death. Further, while our results show that the fluorenone based inhibitors can be useful on their own, an ideal therapy could be to combine them with LF inhibitors to treat late stage anthrax infections, where antibiotics might be unable to prevent death. | ||
| + | <ref>Schein, C.H. et al., Pharmacophore Selection and Redesign of Non-nucleotide Inhibitors of Anthrax Edema Factor. Toxins 2012, 4, 1288-1300; doi:10.3390/toxins4111288</ref> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
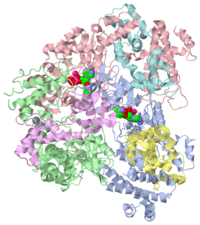
===Crystal structure of the adenylyl cyclase domain of anthrax edema factor (EF) in complex with calmodulin and 2' deoxy, 3' anthraniloyl ATP=== | ===Crystal structure of the adenylyl cyclase domain of anthrax edema factor (EF) in complex with calmodulin and 2' deoxy, 3' anthraniloyl ATP=== | ||
Revision as of 23:56, 5 January 2013
| |||||||||
| 1lvc, resolution 3.60Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Activity: | Adenylate cyclase, with EC number 4.6.1.1 | ||||||||
| Related: | 1k90 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Anthrax edema factor (EF) is an enzyme which is part of the Bacillus anthracis anthrax toxin. Here we study 1lvc – EF adenylate cyclase domain + calmodulin + anthraniloyl-deoxy-ATP.
The edema factor is a calmodulin-dependent adenylate cyclase. Adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] increases intracellular cyclic AMP (cAMP) concentrations in eukaryotic cells. In fact adenylyl cyclases catalyze the conversion of adenosine triphosphate (ATP) into cAMP and pyrophosphate. The cellular level of cAMP increases, upsetting water homeostasis and causing disruption of signaling pathways.
EF is produced in an inactive form. When it is in the cell, EF adenylyl cyclase activity is induced by complexation with calmodulin, so it is allosterically activated. Its enzymatic activity leads to a dramatic elevation of the cAMP range. Calmodulin is ubiquitous eukaryotic cellular protein and a Ca2+ ion sensor present in host cells. Cyclic AMP is a second messenger that plays key roles in the signal transduction pathways and thus regulates diverse cellular responses. It binds to three families of signal transducers: cAMP-dependent protein kinases, cyclic nucleotide gated channels, and the guanine nucleotide exchange factor for Ras GTPase homologs Rap1 and Rap2 (EPAC). [1] [2]
Anthrax toxin
The anthrax toxin is composed of a cell-binding protein (protective antigen) (PA)(83kDa), lethal factor (LF)(90kDa) and edema factor (EF)(89kDa). Edema factor, protective antigen and lethal factor can also be called factor I, II and III respectively. [3] [4]
The complex produced by Bacillus anthracis consists of a virulent mixture of two toxins, with different enzymatic activities. They have a similar N-terminal domain that allows them to bind to a large protein: Protective Antigen.
Protective antigen
PA binds mammalian receptors CMG2 (capillary morphogenesis gene 2) and TEM8 (tumor endothelial marker 8). PA promotes EF and LF translocation in the cytosol of infected cells, particularly macrophages, where the two enzymes perform their damage-inducing processes, allowing bacteria to evade the immune system. [5]
Lethal factor
LF is a zinc-mediated metalloprotease that cleaves mitogen-activated protein kinase kinases (MEKs). This impairs cell signaling, and results in the induction of apoptosis. [6]
Edema factor
EF is a calmodulin-dependent adenylyl cyclase that depletes cellular adenosine triphosphate (ATP) while creating 3',5'-cyclic adenosine monophosphate (cAMP), a cellular second messenger. [7] Both toxins can be reconstituted vitro, by combining PA with LF or EF, to form lethal toxin (LT) or edema toxin (ET).
Edema toxin (ET)
ET is the combination of PA and EF, inducing pathogenic effects. PA is the dominant antigen for immunization, that’s why it is more studied for therapeutic efforts, but LF and EF are also targeted as they are important effectors during anthrax infection.
Associated disease
Bacillus anthracis is the bacterium that is the causative agent of anthrax. The virulence of Bacillus anthracis, a Gram-positive bacterium, is mediated through its poly-D-glutamic acid capsule and its toxin. Bacillus anthracis can form very resistant spores that can survive for decades in the soil and spread easily though water and air. Anthrax is both a disease and bioterrorism threat.
Anthrax is an acute disease, which means that it has a rapid onset and a short course. Most forms of the disease are lethal, and it affects both humans and animals. Anthrax can be contracted in laboratory accidents or by handling infected animals.
Anthrax spores can be produced in vitro and used as a biological weapon to intentionally infect people. As an example, letters containing anthrax spores were mailed to news media offices and U.S. Senators, killing 5 people and infecting 17 others in 2001 in the United States. [8]
Symptoms
Symptoms are depending on the contamination mode. The cutaneous contamination results from a contact of spores with an injury. It leads to an ulcer and to the formation of vesicles. In 80% of the cases, the wound heals without complications. However, sometimes an edem can develop itself and grow. In that case, anthrax can lead to death.
A gastrointestinal contamination can occur because of the consumption of contaminated meat. This form of anthrax leads to ulcers, nauseas, diarrhoea and blood poisoning. It can also be lethal if it is not rapidly treated. Finally, anthrax spores can cause a pulmonary infection by inhalation. The symptoms developed are similar to those of influenza and they evolve into breathing difficulties and hypotension. Blood poisoning and meningitis can also occur. Because of the severe symptoms, the pulmonary infection remains highly lethal. [9] The mortality is caused by the combined effects of bacterial growth (bacteremia) and bacterial toxins (toxaemia).
The secreted proteins can produce two toxic actions. PA associated with LF forms the lethal toxin while PA associated with EF forms the edema toxin. The lethal toxin is involved in the bacterial virulence. The edema toxin plays a key role in anthrax pathogenesis by modulating functions of host cells necessary for immunity. Injection of PA with LF causes death of rats, whereas PA with EF causes edema in the skin of rabbits and guinea pigs. Voir pour le mécanisme d’action si il est connu.
cAMP concentration increase above normal, remain high in the continued presence of toxin, and decrease rapidly after its removal. Since the edema factor secreted by Bacillus anthracis leads to a massive cAMP formation, it affects intracellular signalling pathways. EF may also play a key role in anthrax pathogenesis by disrupting the host cell’s defence against bacterial infection. The toxin has effects on macrophages, dendritic cells, neutrophils, endothelial cells and on the antigen presentation of T cells. For instance, it inhibits the phagocytic activity of neutrophils and alters cytokine production of monocytes by humans. chercher le role de la production de cytokines par les monocytes
Entry of edema toxin in the host cell
The edema factor has a 30 kDa PA-binding domain at its N-terminus. EF PA binding domain can be divides into two subdomains. An N-terminal domain that is composed of three layers at the N-terminus, a/b sandwich domain (four b-sheets, b1–b4, sandwiched by four a-helices, a1–a4) and a domain of five helices at the C-terminus (Figure 1A and C). et le C-t ????? There are five joining loops, L1–L5, where L5 contributes the key residues to bind PA (Figure 1B) chez lf ou chez ef ? Lacy et al, 2002
The edema factor is delivered into host cells thanks to the protective antigen. Indeed the protective antigen binds to cellular receptors (TEM8 or CMP2) and is cleaved at the sequence RKKR by cell surface proteases (non dispo 6). This proteolytic activation leads to the oligomerization of a protective antigen heptamer corresponding to its C-terminal 63 kDa fragment. One heptamer can bind three molecules of edema factor (or lethal factor). Such a complex gets into cells by endocytosis and finally the protective antigen helps the translocation of the edema factor from late endosomes into the cytoplasm. Once it is in the host cell, the edema factor becomes membrane-associated. It is not known whether it is due to its association with calmodulin or to its binding with other cellular elements.
[[A REFORMULER : The surface-exposed residues in L5, a6, a7, and the joining loop between a7 and a8 at the C-terminal five-helix domain of LF-PABD and EF-PABD have been mapped by mutational analysis to make the primary contacts with PA (Lacy et al, 2002). The PA binding surface of EF–PABD is richly negatively charged, which could promote interaction with the positively charged residues at EF/LF interacting surface of PA (Cunningham et al, 2002)]]
The molecular basis of activation and catalysis of the adenylyl cyclase activity of EF by calmodulin
Domain organisation of EF and calmodulin The edema factor has three domains: a PA-binding domain (30 kDa at the N-terminus), a helical domain (17kDa) and acatalytic core domain (43 kDa, in the C-terminal 510 amino acid region) (Drum et al, 2002). The catalytic core domain is itself composed of two domains called CA and CB. The helical domain and the catalytic core are liked by switch C. The adenyly cylase catalytic site is located at the interface of CA and CB. In the absence of calmodulin the enzyme remains inactive thanks to a disordered catalytic loop at the interaction site between the EF helical domain and the catalytic core domain. Calmodulin has two globular domains, the N-terminal and C-terminal domains, that are connected by a flexible a-helix. Each one of these domains can bind two calcium ions thanks to two helix-loop-helix motifs. The binding of a calcium ion induces a conformational change: the domain goes from a hydrophilic “closed” conformation to an “open” state which exposes a hydrophobic pocket. This hydrophobic pocket plays an important role in interaction of calmodulin with other molecules.
The molecular basis for the activation of anthrax EF by calmodulin
........ j'ai pas continué à mettre ta partie, mais je me suis occupée de la mienne :)
Prevention and treatment
It is possible to use classical therapeutic approaches to fight anthrax disease. Since the original vaccine trials by Louis Pasteur in 1881, an attenuated Stern strain is still successfully used as a vaccine in livestock. Many human vaccines based on PA have been developed in the 1960s.
A large number of treatments against anthrax toxin currently in development are antibodies. The majority of these antibodies target the receptor binding domain of PA, blocking binding of PA to cellular receptors. [10]
Efforts to make antibodies to EF have had varied levels of success. Immunoglobulin G (IgG) have been produced, antibodies of moderate affinity to EF, one of which (9F5) was able to inhibit binding of EF to PA and prevent physiological effects of EF on Chinese hamster ovary (CHO) cells. [11]
Winterroth and colleagues described six antibodies of moderate affinity, including one IgM that could neutralize EF activity in CHO cells. This antibody had no significant protective effect against a Sterne strain infection in a mouse model but extended the mean time to death when combined with a subprotective dose of anti-PA antibody. [12]
Chen and colleagues developed chimpanzee antibodies that included one having very high affinity and that competes with calmodulin for binding to the helical domain of EF. This antibody was very effective at preventing ET-mediated edema in a mouse footpad model and provided significant protection in a systemic toxin challenge. [13]
Leysath et al. developed and characterized four anti-EF monoclonal antibodies (MAb). MAb bind to epitopes on three different domains of EF: PA binding domain, catalytic CB domain, and helical domain. They showed that three of the four IgGs have neutralizing activity in vitro and in vivo, inhibiting production of cAMP. Antibody 7F10 has the highest efficacy and 3F2 is the first monoclonal antibody to EF whose epitope is on the catalytic CB domain. MAb are highly specific for EF and do not cross-react with LF or PA and can decrease edema levels and progression of disease in mouse models. These antibodies can serve as reagents in diagnostics assays, and can be useful reagents for further molecular studies of EF action. [14]
Inhibitors
Schein et al. identified non-nucleotide inhibitors of EF. Inhibitors targeting sites for such allosteric activators have recently been identified. [15] They chose to do a direct design based on analysis of the structure of the substrate binding site of the EF protein, rather than a design starting from modifying nucleotides related to the substrate itself. Their studies focused on the active site (circled in the structure of EF bound to calmodulin, shown in Figure 1Top). Comparison of the active site conformation in various crystal structures in the Protein database (PDB) (which differed in the number and types of bound metal ions and substrates [16]) revealed important information about how the active site of the toxin differed from the mammalian adenyl cyclase enzymes. These crystal structures, with or without the bound metal ions, were used for docking potential inhibitors identified by our fragment based pharmacophore. Our previous studies [16] indicated that the docking of 3'-dATP to the crystal structure of EF (PDB structure: 1K90 [16] had low RMSD (root-mean-square deviations) between the predicted structure and the crystal structure, and thus, AutoDock 3.0 was reliable enough to predict the binding mode of the ligands to EF. Although early treatment with antibiotics can greatly reduce the effects of bacterial infections, inhibitors of the toxins could play a therapeutic role in later stage infections, in preventing toxin induced diarrhea or even death. Further, while our results show that the fluorenone based inhibitors can be useful on their own, an ideal therapy could be to combine them with LF inhibitors to treat late stage anthrax infections, where antibiotics might be unable to prevent death. [17]
Crystal structure of the adenylyl cyclase domain of anthrax edema factor (EF) in complex with calmodulin and 2' deoxy, 3' anthraniloyl ATP
Edema factor (EF) and CyaA are calmodulin (CaM)-activated adenylyl cyclase exotoxins involved in the pathogenesis of anthrax and whooping cough, respectively. Using spectroscopic, enzyme kinetic and surface plasmon resonance spectroscopy analyses, we show that low Ca(2+) concentrations increase the affinity of CaM for EF and CyaA causing their activation, but higher Ca(2+) concentrations directly inhibit catalysis. Both events occur in a physiologically relevant range of Ca(2+) concentrations. Despite the similarity in Ca(2+) sensitivity, EF and CyaA have substantial differences in CaM binding and activation. CyaA has 100-fold higher affinity for CaM than EF. CaM has N- and C-terminal globular domains, each binding two Ca(2+) ions. CyaA can be fully activated by CaM mutants with one defective C-terminal Ca(2+)-binding site or by either terminal domain of CaM while EF cannot. EF consists of a catalytic core and a helical domain, and both are required for CaM activation of EF. Mutations that decrease the interaction of the helical domain with the catalytic core create an enzyme with higher sensitivity to Ca(2+)-CaM activation. However, CyaA is fully activated by CaM without the domain corresponding to the helical domain of EF.
Physiological calcium concentrations regulate calmodulin binding and catalysis of adenylyl cyclase exotoxins., Shen Y, Lee YS, Soelaiman S, Bergson P, Lu D, Chen A, Beckingham K, Grabarek Z, Mrksich M, Tang WJ, EMBO J. 2002 Dec 16;21(24):6721-32. PMID:12485993
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
1lvc is a 6 chain structure with sequence from Bacillus anthracis and Homo sapiens. Full crystallographic information is available from OCA.
See Also
Reference
- ↑ Fouet, A. 2009. The surface of Bacillus anthracis. Mol. Aspects Med. 30:374–385
- ↑ Moayeri, M., and S. H. Leppla. 2009. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 30:439–455
- ↑ Fouet, A. 2009. The surface of Bacillus anthracis. Mol. Aspects Med. 30:374–385
- ↑ Moayeri, M., and S. H. Leppla. 2009. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 30:439–455
- ↑ Tournier, J.N.; Rossi Paccani, S.; Quesnel-Hellmann, A.; Baldari, C.T. Anthrax toxins: A weapon to systematically dismantle the host immune defenses. Mol. Aspects Med. 2009, 30, 456–466
- ↑ Klimpel, K. R., N. Arora, and S. H. Leppla. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 13:1093–1100
- ↑ Leppla, 1982, Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cAMP concentration in eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3163
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3064363/
- ↑ http://en.wikipedia.org/wiki/Anthrax_toxin
- ↑ Young, J. A., and R. J. Collier. 2007. Anthrax toxin: receptor-binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76:243–265
- ↑ Little, S. F., S. H. Leppla, J. W. Burnett, and A. M. Friedlander. 1994. Structure-function analysis of Bacillus anthracis edema factor by using monoclonal antibodies. Biochem. Biophys. Res. Commun. 199:676–682
- ↑ Winterroth, L., J. Rivera, A. S. Nakouzi, E. Dadachova, and A. Casadevall. 2010. A neutralizing monoclonal antibody to edema toxin and its effect on murine anthrax. Infect. Immun. 78:2890–2896
- ↑ Chen, Z., et al. 2009. Potent neutralization of anthrax edema toxin by a humanized monoclonal antibody that competes with calmodulin for edema factor binding. Proc. Natl. Acad. Sci. U. S. A. 106:13487–13492
- ↑ Leysath, C.E. et al., Mouse Monoclonal Antibodies to Anthrax Edema Factor Protect against Infection. Infection and immunity, Nov. 2011, p. 4609–4616
- ↑ Laine, É.; Martínez, L.; Ladant, D.; Malliavin, T.; Blondel, A. Molecular motions as a drug target: Mechanistic simulations of anthrax toxin edema factor function led to the discovery of novel allosteric inhibitors. Toxins 2012, 4, 580–604
- ↑ Drum, C.L.; Yan, S.Z.; Bard, J.; Shen, Y.Q.; Lu, D.; Soelaiman, S.; Grabarek, Z.; Bohm, A.; Tang, W.J. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature 2002, 415, 396–402
- ↑ Schein, C.H. et al., Pharmacophore Selection and Redesign of Non-nucleotide Inhibitors of Anthrax Edema Factor. Toxins 2012, 4, 1288-1300; doi:10.3390/toxins4111288