Sandbox Reserved 714
From Proteopedia
| Line 64: | Line 64: | ||
As many enzymes, the human soluble epoxide hydrolase can be inhibited by some inhibitors, causing a loss of activity for the enzyme. | As many enzymes, the human soluble epoxide hydrolase can be inhibited by some inhibitors, causing a loss of activity for the enzyme. | ||
| - | The sEH can be inhibited by some metal ions such as Zn<sup>2+</sup>, Cu<sup>2+</sup>, Hg<sup>2+</sup>. It is a noncompetitive inhibition: the metal ion binding to the enzyme reduces its activity without affecting directly the substrate binding, so that the V<sub>max</sub> decreases but the K<sub>m</sub> remains unchanged. It may be that those metal ions replace the Mg<sup>2+</sup> in the N-term active site of the enzyme subunits, which can result in some conformation changes, but this is only an hypothesis. | + | The sEH can be inhibited by some metal ions such as Zn<sup>2+</sup>, Cu<sup>2+</sup>, Hg<sup>2+</sup> <ref name="EH" />. It is a noncompetitive inhibition: the metal ion binding to the enzyme reduces its activity without affecting directly the substrate binding, so that the V<sub>max</sub> decreases but the K<sub>m</sub> remains unchanged. It may be that those metal ions replace the Mg<sup>2+</sup> in the N-term active site of the enzyme subunits, which can result in some conformation changes, but this is only an hypothesis. |
However, there are also some chemical inhibitors that inhibit the sEH. They are 1-3 disubstitued ureas, carbamates and amides, which are stable inhibitors for the sEH. | However, there are also some chemical inhibitors that inhibit the sEH. They are 1-3 disubstitued ureas, carbamates and amides, which are stable inhibitors for the sEH. | ||
Revision as of 23:59, 5 January 2013
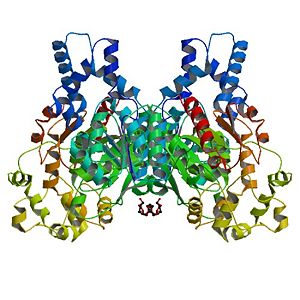
The Human soluble Epoxyde Hydroxylase (hsEH) is a homodimeric protein as well as a bifunctional enzyme, which is made of 555 aminoacids and can be found in the cytoplasm and in peroxisomes of humans. It belongs to the “Hydrolase” enzyme family. This enzyme has two functions: an epoxide hydrolase function on its C-term domain, which consists in the hydrolysis of epoxides into glycols, and a phosphatase function on its N-term domain, which consists in cleaving a phosphate group from a dihydroxy lipid phosphate.
Contents |
Biological role
This enzyme is expressed in mammalian liver, vascular endothelium, proximal tubule and some smooth muscles. By hydrolyzing epoxides and lipid phosphates, it has a role in detoxification and it prevents DNA from alkylation by those epoxides. At the physiological level, hsEH participates to the regulation of cardiovascular and renal physiology and to inflammatory biology as well[1]. More specifically, this enzyme is a regulator of mammalian blood pressure and could be a target for a treatment of hypertension[2]. Thanks to mutagenesis experiments[3], it has been demonstrated that the inhibition of sEH lowers blood pressure.
| |||||||||||
Additional 3D Structures of hsEH
1vj5 - hsEH + N-cyclohexyl-N'-(4-iodophenyl)urea complex
1zd2,1zd3, 1zd4,1zd5 - hsEH + 4-(3-cyclohexyluriedo)-carboxylic acids
3ant - Hydrolase domain + synthetic inhibitor
3pdc - Hydrolase domain + benzoxazole inhibitor
External ressources
Protein Data Bank entry on 1S8O
Uniprot link on Bifunctional epoxyde hydrolase 2
Wikipedia page on Epoxyde hydrolase 2
References
- ↑ Gomez GA, Morisseau C, Hammock BD, Christianson DW. Human soluble epoxide hydrolase: structural basis of inhibition by 4-(3-cyclohexylureido)-carboxylic acids. Protein Sci. 2006 Jan;15(1):58-64. Epub 2005 Dec 1. PMID:16322563 doi:10.1110/ps.051720206
- ↑ Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000 Dec 22;275(51):40504-10. PMID:11001943 doi:10.1074/jbc.M008106200
- ↑ Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002 Feb;39(2 Pt 2):690-4. PMID:11882632
- ↑ Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311-33. PMID:15822179 doi:10.1146/annurev.pharmtox.45.120403.095920
- ↑ Gomez GA, Morisseau C, Hammock BD, Christianson DW. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry. 2004 Apr 27;43(16):4716-23. PMID:15096040 doi:10.1021/bi036189j
- ↑ Newman JW, Morisseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1558-63. Epub 2003 Feb 6. PMID:12574510 doi:10.1073/pnas.0437724100
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedEH
Proteopedia Page Contributors and Editors
DUTREUX Fabien, BONHOURE Anna

