This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 716
From Proteopedia
m |
|||
| Line 17: | Line 17: | ||
When the P-selectin is activated by molecules like histamine or thrombin, it is able to bind myeloid cells and some T cells. | When the P-selectin is activated by molecules like histamine or thrombin, it is able to bind myeloid cells and some T cells. | ||
| - | |||
== '''3D structure''' == | == '''3D structure''' == | ||
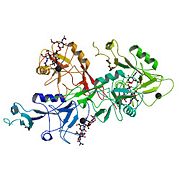
| - | < | + | <StructureSection load='1g1r' size='400' side='left' caption='Structure of cholesteryl ester tranfer protein (PDB entry [[1g1r]])' scene=''> |
| - | + | ||
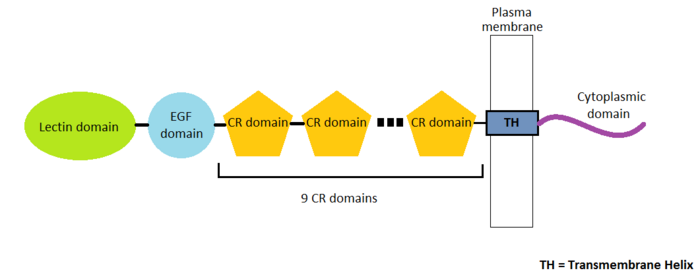
P-Selectin is a protein composed of 4 different chains A, B, C and D containing 162 amino-acids residues. There are many domains in this protein. Three types of domains represent the extracellular part of the protein : the '''EGF domain''', the '''lectin domain''' and nine '''consensus repeat (CR) domains'''. Finally, we find a transmembrane helix and a cytoplasmic domain.<ref>PMID 11081633</ref> | P-Selectin is a protein composed of 4 different chains A, B, C and D containing 162 amino-acids residues. There are many domains in this protein. Three types of domains represent the extracellular part of the protein : the '''EGF domain''', the '''lectin domain''' and nine '''consensus repeat (CR) domains'''. Finally, we find a transmembrane helix and a cytoplasmic domain.<ref>PMID 11081633</ref> | ||
| Line 76: | Line 74: | ||
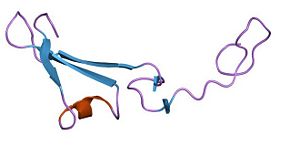
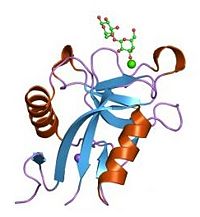
| + | Lectin domains (also known as C-type lectin domains) are classified in 17 groups (from I to XVII). P-Selectin lectin domain bellow to the group IV. | ||
| + | <scene name='Sandbox_Reserved_716/Lectin_domain/2'>P-selectin lectin domain</scene> is composed by 118 amino-acids residues and is a succesion of β-sheets and helix. | ||
| + | It binds (via an extended site of the domain) a ligand : '''the carbohydrate sialyl LewisX (SLeX)''' with low affinity. However, the affinity between the ligand and the selectins is actually quite high. A theory may explain why lectin domain may recognize SLeX (or related carbohydrate structures on this ligand) with a higher affinity. Indeed EGF domain may alter the conformation of the lectin domain so that its affinity for the ligand is increased. | ||
| - | Lectin domains (also known as C-type lectin domains) are classified in 17 groups (from I to XVII). P-Selectin lectin domain bellow to the group IV. | ||
| - | < | + | </StructureSection> |
| + | |||
| + | |||
| - | It binds (via an extended site of the domain) a ligand : '''the carbohydrate sialyl LewisX (SLeX)''' with low affinity. However, the affinity between the ligand and the selectins is actually quite high. A theory may explain why lectin domain may recognize SLeX (or related carbohydrate structures on this ligand) with a higher affinity. Indeed EGF domain may alter the conformation of the lectin domain so that its affinity for the ligand is increased. | ||
Revision as of 12:36, 6 January 2013
Crystal structure of P-selectin lectin/EGF domains complexed with SLeX
Contents |
Introduction
Selectins are proteins that are include in a family of cell adhesion receptor involved in the leukocyte extravasation. There are 3 kinds of selectins : E selectin localized in endothelial cells, L selectin found in leukocytes, and P selectins in platelets and endothelial cells.
In this page we will be focused only on P-Selectin.
When the P-selectin is activated by molecules like histamine or thrombin, it is able to bind myeloid cells and some T cells.
3D structure
| |||||||||||
Different role of the P-selectin
Role in leukocyte extravasation
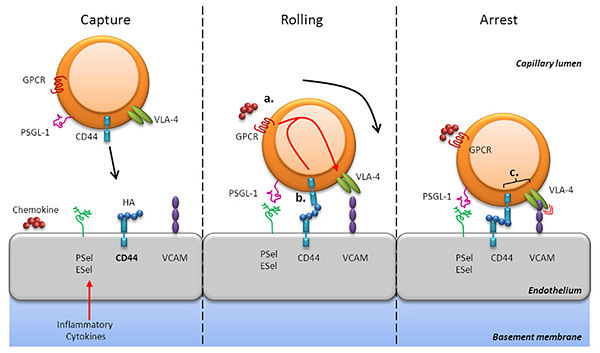
Leukocyte extravasation is the movement of leukocytes out of the circulatory system at the time of an infection. The inflammation is a natural reaction of defense caused by tissular damages. The recruitement of leukocytes on the inflammation site is a process in four steps. First, the leukocyte is attracted by cytokines, secreted near the site of infection (these cytokines are also able to activate P-selectins). This is the chemoattraction. Then, this leukocyte begin rolling along the inner surface of the vessel, binding on selectin molecules. The third step is the tight adhesion, the leukocyte immobilizates himself, thanks to the integrin molecules. And finally, he passes through gaps between endothelial cells. This last step is not shown in the following drawing. By this mecanism, the leukocyte arrives on the site of infection to neutralize the infection agent.
Role in platelets recruitment
At areas of vascular injury, P-selectin recruits and aggregates platelets[3] through platelet-fibrin and platelet-platelet binding. In quiescent platelet, P-selectin is located on the inner wall of α-granules. The activation of platelets caused by thrombin, Type II collagen and ADP results in "membrane flipping" where the platelet releases α- and dense granules and the inner walls of the granules are exposed on the outside of the cell.
Role in cancer
P-selectin also has a functional role in formation of tumor. P-selectin is expressed on the surface of both stimulated endothelial cell and activated platelet and helps cancer cells invade into bloodstream for metastasis. Moreover, platelet facilitates tumor metastasis by forming complexes with tumor cells and leukocytes. In vivo mice experiment showed that reduction in circulating platelets could reduce cancer metastasis. (source) development of new compounds that target P-selectin is now emerging for cancer therapy
External resources
Protein Data Bank file 1G1R
References
- ↑ Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000 Oct 27;103(3):467-79. PMID:11081633
- ↑ Kansas GS, Saunders KB, Ley K, Zakrzewicz A, Gibson RM, Furie BC, Furie B, Tedder TF. A role for the epidermal growth factor-like domain of P-selectin in ligand recognition and cell adhesion. J Cell Biol. 1994 Feb;124(4):609-18. PMID:7508943
- ↑ Phan UT, Waldron TT, Springer TA. Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat Immunol. 2006 Aug;7(8):883-9. Epub 2006 Jul 16. PMID:16845394 doi:10.1038/ni1366
Proteopedia Page Contributors and Editors
Delphine Trelat, Cécile Ehrhardt