1wco
From Proteopedia
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
| - | The emerging antibiotics-resistance problem has underlined the urgent need | + | The emerging antibiotics-resistance problem has underlined the urgent need for novel antimicrobial agents. Lantibiotics (lanthionine-containing antibiotics) are promising candidates to alleviate this problem. Nisin, a member of this family, has a unique pore-forming activity against bacteria. It binds to lipid II, the essential precursor of cell wall synthesis. As a result, the membrane permeabilization activity of nisin is increased by three orders of magnitude. Here we report the solution structure of the complex of nisin and lipid II. The structure shows a novel lipid II-binding motif in which the pyrophosphate moiety of lipid II is primarily coordinated by the N-terminal backbone amides of nisin via intermolecular hydrogen bonds. This cage structure provides a rationale for the conservation of the lanthionine rings among several lipid II-binding lantibiotics. The structure of the pyrophosphate cage offers a template for structure-based design of novel antibiotics. |
==About this Structure== | ==About this Structure== | ||
| - | 1WCO is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Lactococcus_lactis Lactococcus lactis] and [http://en.wikipedia.org/wiki/Monarthropalpus_flavus Monarthropalpus flavus] with <scene name='pdbligand=NAG:'>NAG</scene>, <scene name='pdbligand=MUB:'>MUB</scene> and <scene name='pdbligand=FPP:'>FPP</scene> as [http://en.wikipedia.org/wiki/ligands ligands]. This structure | + | 1WCO is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Lactococcus_lactis Lactococcus lactis] and [http://en.wikipedia.org/wiki/Monarthropalpus_flavus Monarthropalpus flavus] with <scene name='pdbligand=NAG:'>NAG</scene>, <scene name='pdbligand=MUB:'>MUB</scene> and <scene name='pdbligand=FPP:'>FPP</scene> as [http://en.wikipedia.org/wiki/ligands ligands]. This structure supersedes the now removed PDB entry 1UZT. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1WCO OCA]. |
==Reference== | ==Reference== | ||
| Line 14: | Line 14: | ||
[[Category: Monarthropalpus flavus]] | [[Category: Monarthropalpus flavus]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| - | [[Category: Bonvin, A | + | [[Category: Bonvin, A M.J J.]] |
[[Category: Breukink, E.]] | [[Category: Breukink, E.]] | ||
| - | [[Category: Hsu, S | + | [[Category: Hsu, S T.D.]] |
[[Category: Kaptein, R.]] | [[Category: Kaptein, R.]] | ||
| - | [[Category: Kruijff, B | + | [[Category: Kruijff, B De.]] |
| - | [[Category: Lutters, M | + | [[Category: Lutters, M A.G.]] |
| - | [[Category: Nuland, N | + | [[Category: Nuland, N A.J Van.]] |
[[Category: Tischenko, E.]] | [[Category: Tischenko, E.]] | ||
[[Category: FPP]] | [[Category: FPP]] | ||
| Line 30: | Line 30: | ||
[[Category: pyrophosphate cage]] | [[Category: pyrophosphate cage]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 15:42:52 2008'' |
Revision as of 13:42, 21 February 2008
|
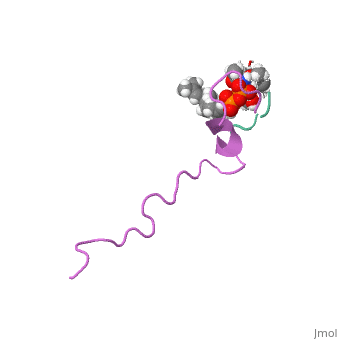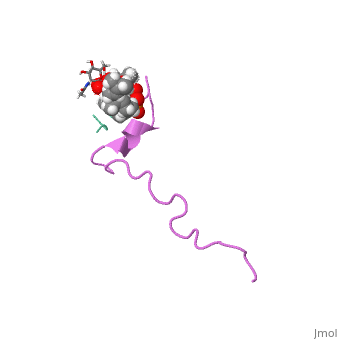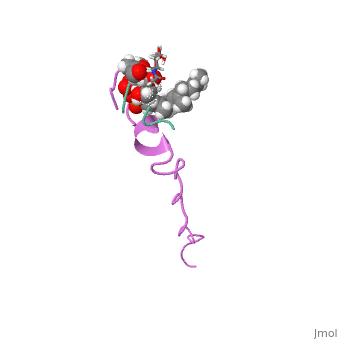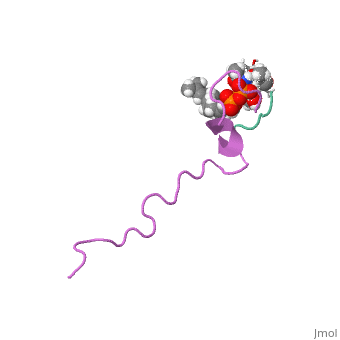
THE SOLUTION STRUCTURE OF THE NISIN-LIPID II COMPLEX
Overview
The emerging antibiotics-resistance problem has underlined the urgent need for novel antimicrobial agents. Lantibiotics (lanthionine-containing antibiotics) are promising candidates to alleviate this problem. Nisin, a member of this family, has a unique pore-forming activity against bacteria. It binds to lipid II, the essential precursor of cell wall synthesis. As a result, the membrane permeabilization activity of nisin is increased by three orders of magnitude. Here we report the solution structure of the complex of nisin and lipid II. The structure shows a novel lipid II-binding motif in which the pyrophosphate moiety of lipid II is primarily coordinated by the N-terminal backbone amides of nisin via intermolecular hydrogen bonds. This cage structure provides a rationale for the conservation of the lanthionine rings among several lipid II-binding lantibiotics. The structure of the pyrophosphate cage offers a template for structure-based design of novel antibiotics.
About this Structure
1WCO is a Single protein structure of sequence from Lactococcus lactis and Monarthropalpus flavus with , and as ligands. This structure supersedes the now removed PDB entry 1UZT. Full crystallographic information is available from OCA.
Reference
The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics., Hsu ST, Breukink E, Tischenko E, Lutters MA, de Kruijff B, Kaptein R, Bonvin AM, van Nuland NA, Nat Struct Mol Biol. 2004 Oct;11(10):963-7. Epub 2004 Sep 12. PMID:15361862
Page seeded by OCA on Thu Feb 21 15:42:52 2008