Group:MUZIC:Myostatin
From Proteopedia

(→Sequence annotation) |
|||
| Line 8: | Line 8: | ||
== Sequence annotation == | == Sequence annotation == | ||
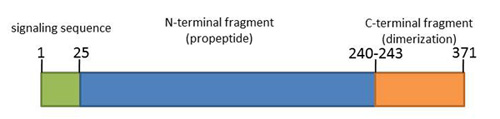
Myostatin consists of three functional fragments: signal peptide, N-terminal and C-terminal fragments. Amino acid sequences among vertebrate species are highly conserved, which also suggests the conserved function of myostatin among vertebrates <ref>PMID: 9356471 </ref>. | Myostatin consists of three functional fragments: signal peptide, N-terminal and C-terminal fragments. Amino acid sequences among vertebrate species are highly conserved, which also suggests the conserved function of myostatin among vertebrates <ref>PMID: 9356471 </ref>. | ||
| - | + | [[Image:myostatin.jpg]] | |
== Structure == | == Structure == | ||
Revision as of 11:14, 11 February 2013
|
Contents |
Introduction
Myostatin which is also known as growth and developmental factor-8(GDF-8) was originally identified in a screen for novel mammalian members of the transforming growth factor-ß (TGF-ß) superfamily of growth and differentiation factors.The phenotype of myostatin knock-out mice suggested that myostatin functions as a negative regulator of muscle growth, and it was on this basis that myostatin was given its name [1]. For these reasons, inhibitors targeting myostatin have been regarded as potential drugs in the treatment of muscle-wasting disorders such as muscular dystrophy [2].
Sequence annotation
Myostatin consists of three functional fragments: signal peptide, N-terminal and C-terminal fragments. Amino acid sequences among vertebrate species are highly conserved, which also suggests the conserved function of myostatin among vertebrates [3].
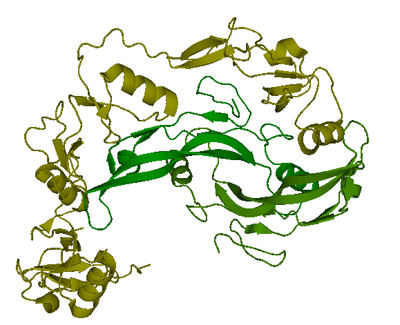
Structure
One structure of myostatin is currently available in Protein Data Bank. The complex of two follistatin 288 molecules bound to one myostatin dimer was resolved to 2.15 Å using X-ray crystallography and deposited in PDB. (Green:myostatin C-terminal dimer, yellow: follinstatin 288)
Function and Interactions
Myostatin is initially formed as a precursor protein which undergoes two proteolytic processing events in order to generate the biologically active molecule. First the N-terminal signal sequence is removed, a second cleavage generates the C-terminal fragment, which possesses receptor-binding activity and modulates a signal transduction cascade in the target cell [4]. The N-terminal fragment after proteolytic processing has been referred to as the propeptide (shown blue). One mechanism for activating myostatin latency appears to be proteolytic cleavage of the propeptide [5]. In addition to the regulation of intracellular myostation processing,follistatin has been known to be capable of binding and inhibiting the activity of the myostatin C-terminal dimer (shown yellow). [6].
The interaction of myostatin with titin-cap(T-cap),a Z-disk protein which binds to N-terminal domain of titin,was identified by a yeast two-hybrid system. [7] It is presumed that myostatin has a putative role in the muscle Z-disk regulation.
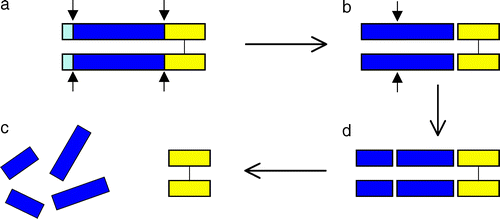 (Processing of myostatin protein. Lee,2004)
(Processing of myostatin protein. Lee,2004)
Pathology
Mice lacking C-terminal domain of myostatin shows dramatic increases in skeletal muscle mass, which are observed for the entire life of mice. In addition, myostatin mutant mice fail to accumulate fat as a function of age and suppress the development of insulin resistance [8]. Thus, the targeting myostatin pathway might be an effective way to promote muscle growth for the patients with muscle degenerative diseases, such as muscular dystrophy and to prevent obesity [9].
References
- ↑ McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997 May 1;387(6628):83-90. PMID:9139826 doi:10.1038/387083a0
- ↑ Bradley L, Yaworsky PJ, Walsh FS. Myostatin as a therapeutic target for musculoskeletal disease. Cell Mol Life Sci. 2008 Jul;65(14):2119-24. PMID:18425412 doi:10.1007/s00018-008-8077-3
- ↑ McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997 Nov 11;94(23):12457-61. PMID:9356471
- ↑ Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61-86. PMID:15473835 doi:10.1146/annurev.cellbio.20.012103.135836
- ↑ Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15842-6. Epub 2003 Dec 11. PMID:14671324 doi:10.1073/pnas.2534946100
- ↑ Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990 Feb 16;247(4944):836-8. PMID:2106159
- ↑ Nicholas G, Thomas M, Langley B, Somers W, Patel K, Kemp CF, Sharma M, Kambadur R. Titin-cap associates with, and regulates secretion of, Myostatin. J Cell Physiol. 2002 Oct;193(1):120-31. PMID:12209887 doi:10.1002/jcp.10158
- ↑ McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002 Mar;109(5):595-601. PMID:11877467 doi:10.1172/JCI13562
- ↑ Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61-86. PMID:15473835 doi:10.1146/annurev.cellbio.20.012103.135836