|
Introduction to IgA
The most extensive surface in contact with the external environment is not our skin, but the epithelial lining of our gastrointestinal, respiratory, and urogenital tracts [1]. As a first line of defense in maintenance the integrity our mucosa, the immune system manufactures and secretes dimeric IgA to neutralize pathogenic organisms [2] and exclude the entry of commensals at the mucosal border [3]. In the serum, IgA functions as a second line of defense against pathogens that may breech the epithelial boundary [2]. The body produces more IgA than any other antibody isotype [3]. In fact, IgA is the most abundant antibody in the body, further illustrating IgA's critical role in immunity [4].
At least two isotypes exist, termed IgA1 and IgA2. IgA2 can further be categorized into 2 allotypes: IgA2 m(1) and IgA2 m(2). While IgA2 is found in most mammalian species, IgA1 is found only in higher apes. An approximately equal ratio of secretory IgA1 (sIgA1) to secretory IgA2 (sIgA2) reside at the mucosal surface, with the exception of the colon, where the majority is sIgA2 [5]. In the serum, about 90% of the IgA is monomeric IgA1 [4]. While both isoforms are able to bind polysaccharide, IgA1 preferentially binds protein antigen, while IgA2 preferentially binds lipopolysaccharide lipid A.
The receptors for IgA include the Fcα Receptor (FcαRI; CD89) and the polyimmunologlobulin receptor (pIgR). When binding to FcαRI results in the dimerization, the consequent signaling results in effector functions, including respiratory burst, mucosal surface, phagocytosis, and eosinophil degranulation. Binding to the pIgR results in transocytosis and IgA secretion [2]. Unlike other antibody isotypes, IgA exists in multiple oligomeric states [3]. The most common of which are the monomeric, dimeric, and secretory forms [4], adding to the complexity of structural functions for IgA. Exploring IgA's structure and protein interactions illuminates the unique and critical function IgA plays in humoral immunity.
Antibody Structure and the Immunoglobulin Domain
Overall Structure
- An antibody is a tetramer of and . In other words, the antibody is a of 2 heterodimers. Each is comprised on one light chain and one heavy chain. Heavy and light chains are held together with disulfide bonds and noncovalent interactions.
Fab and Fc fragments
- Another common way of describing antibody structure is in terms of its Fab and Fc fragments. Each light chains are composed of 2 immunoglobulin domains: one variable domain</scene> and one constant domain. Heavy chains composed of 4 Ig domains: one V-type and 3 C-type, named CH1 - CH3. A linking hinge region separates the CH2 and CH3 domains. Proteolytic cleavage at the hinge region by the protease papain, or a similar protease, yields 2 Fab fragments and 1 Fc fragment. Each contains 2 variable domains, one from the heavy chain and one from the light chain, and 2 constant domains one from the light chain and the Ch1 domain from the heavy chain. The Fc fragment contains 4 constant domains: the Ch2 and Ch3 domains from each of the heavy chains. Since the variable portions determine antigen specificity, the Fab fragments are generally thought of as the antigen-binding portion. The Fc fragment is important in binding various receptors, many of which are isotype specific and are named after the isotype of the ligand, i.e. FcαR binds the Fc portion of IgA.
Immunoglobulin domains
- The antibody is a member of the immunoglobulin superfamily of proteins [6]. Each chain can be further broken down into immunoglobulin domains: 2 in the light chain and 4 in the heavy chain, for a total of 12 in the entire antibody. Each immunoglobulin domain contains a primary amino acid sequence of approximately 70 – 100 residues long. Secondary structure is a characteristic beta sandwich with a variable number of beta strands, depending on the unit type. These strands display Greek key connectivity (web other) and form 2 beta sheets that fold over each other. An intra-domain disulfide bond stabilizes the tertiary structure.
-
- Nine antiparallel beta strands comprise variable or V-regions. Loop sequences of varying length connect the strands. The 9 strands form 2 beta sheets, one with 4 (ABED-prosite) strands and the other with 3 sIgA2(nov 22 2007) [7]. The remaining 2 strands (C’ and C”) lie in between the 2 sheets. A disulfide bride stabilizes the 2 sandwich halves. Hydrophobic residues face the interior of the sheet, providing stability, while hydrophilic residues face outward and interact with the local environment. The extra loops in the V-region are critical for epitope specificity, and are consequently known as the compliment determining regions, here shown on the .
-
- C-type domains lack the C' and C'' beta strands [6]. The sheets are ABED and CFG. Consequently, the sandwich is more tightly packed. In the antibody, the constant domains determine the isotype: IgA, IgD, IgM, IgG, or IgE.
- Related structures
- Proteins containing the classic immunoglobulin-like domain are found predominantly in the immune system [6]. In fact, the antibody's closest related structures are those that recognize antigen: MHC and TCRs.
- The V-type domain is found in a wider variety of proteins, including the Ig-binding molecules, such as the pIgR and the FcαR [6]. Viral hemagluttinin is yet another example.
IgA1 and IgA2: a Structural Comparison
Hinge Region
- The hinge region differs significantly between the two IgA isoforms [2]. The hinge region of IgA1 is comprised of 23 residues (PVPSTPPTPSPSTPPTPSPSCCH) and 5 O-glycosylation sites, while IgA2’s hinge region is comprised of 10 residues (PVPPPPPCCH) and no sites of glycosylation. Both hinge regions are located at Cys220 on the Ch1 chain and end at Ch2’s Pro244; however, the naming system is misleading, as it follows IgA1 and is therefore misleading. In fact, the distance from the center of the 2 Fab fragments in IgA1 is 16.9nm versus 8.2 nm in IgA2. So, while IgA1 remains extended, IgA2 is more compact. The greater number of residues in the IgA1 hinge region corresponds to a greater antigenic reach.
- These data must be taken into account with other hinge region characteristics [2]. IgA1’s hinge region contains 5 sites of O-glycosylation, while IgA2’s hinge region contains none. In addition, IgA1’s hinge region contains 10 Pro residues, while IgA2’s region contains 6. In comparison, IgG’s hinge region contains No glycine residues reside in the hinge regions of either IgA1 or IgA2. The presence of prolines, the absence of glycine and the presence of glycosylated residues in IgA1 all amount to increased hinge rigidity in comparison to IgG1.
N-glycosylation
- In the harsh mucosal environment, glycosylated residues protect the protein from proteases [2]. Both IgA1 and IgA2 display N-glycosylated residues. IgA1 has 3, at N263 on beta strand B on the Ch2 chain and on the J tail at N459. In IgA2, additional sites of N-glycosylation include Asn166 on the beta strand G of Ch1 and Asn337 of beta strand G on Ch2. Some alloforms of IgA2 are also N-glycosylated at Asn211 on Ch2. An increased need for protection against proteolytic cleavage at the hinge region accounts for the presence of O-glycosylation in IgA1’s hinge region, particularly cleavage by bacterial metalloproteases. The glycosylation residues provide increased steric hindrance, and creating difficulty in fitting the peptide in the protease’s active site. In comparison to IgG, which is only 2.9% (w/w) glycosylated, IgA1 is 9.5% (w/w) and IgA2 is 11% (w/w) glycosylated. Overall, IgA1 is more susceptible to proteases than IgA2.
Disulfide Bonds
- The two structures also differ in the locations of their disulfide bonds [2]. In IgA1, a disulfide bond exists between the heavy chain Cys220 and light chain Cys196. This disulfide bond is absent in the main form of IgA2. Instead a disulfide bond links the 2 light chains at their C termini. The heavy and light chain associate through noncovalent interactions. So, while IgA1 may be more susceptible to proteases, IgA2 is more susceptible to denaturing conditions.
T-shape
- The unique characteristics of IgA1 and IgA2 explain the antibodies' overall T-shape [2]. IgA distinctly lacks the classic "Y-shape" antibody structure. IgA's increased hinge rigidity and a longer hinge region result in IgA1's predominately T-shape, in comparison to IgG's Y-shape. While the structure of IgA2 is more compact, the combination of an inter-light chain disulfide bond, a short hinge region, and proline residues with the hinge provide steric forces compatible with a T-shape. Of note, the T-shaped IgA2, with its interchain disulfide bond, resembles the structure of an IgG lacking the disulfide bonds between the heavy and light chains, which suggests the possibility of an evolutionary relationship between the two. The presence of IgA2 in lower mammals in contrast to IgA1 also supports this hypothesis.
Compare and Contrast
IgA1
- Protect from proteases and increase hinge rigidity. Note the extended hinge region of 23 amino acids, extending IgA1's antigenic reach.
- Protect from proteases and increase hinge rigidity.
- planar (fab fragments aligned with Fc portion)
IgA2
- Protect from proteases and increase hinge rigidity.
- nonplanar (fab fragments not aligned with Fc portion)
IgG
- Y shaped, with an intermediate length hinge region.
- increase hinge flexibility. There are no proline residues in IgG's hinge region.
- (missing one fab fragment. Note the T-shape. Compare with IgA2.
- Hinge region is 64 amino acids in length. Note similarity to IgA1.
The J Chain allows IgA to form Dimers
- The IgA structure has an addition 18 kDa, 137 residue polypeptide chain called the [4]. This 18 kDa, 137-residue polypeptide chain is comprised of 2 immunoglobulin-like domains. The J chain is covalently attached to the C terminal Cys471 on IgA's Ch3 domain [8] via a disulfide bridge with either the J chain’s Cys 14 or the Cys 68 [4], [8]. The J chain has a single N-linked oligosaccharide 15111057, which increases rigidity and offers protection against proteases. The J chain allows IgA to form , and less often trimer and tetramers. These polymers are rare because steric hindrance from the T-shaped Fab regions makes polymerization thermodynamically unfavorable.
- When IgA forms dimers, the Fc regions align end to end without overlap [8]. The J chain lies within a fold in the bent Fc region. This conformation may allow the J chain access to the Secretory Component of the pIgR, which allows translocation across the mucosal epithelia to the luminal surface. Of note, in the image the J chains the J chains are extending from the dimer, which does not match with the described interaction of the J chain with the Fc portions of the antibody (see Limitations of the Current Studies).
Secretory Component
- IgA is secreted as a dimer when it binds to the pIgR and is transported across the cell membrane [4]. Upon IgA binding, the receptor-antibody complex is transocytosed to the lumenal side, where native proteases cleave the pIgR, releasing the secretory IgA (sIgA) into the lumen. The region of the pIgR that remains attached to the IgA upon pIgR cleavage is known as the .
- The secretory component is the first 585 residues of the pIgR [1]. The C terminal end of the secretory component is linked to the pIgR, but maintains no specific fold. The ability for the secretory to move freely facilitates its proteolytic cleavage and the secretion of sIgA. Structurally, the secretory component is comprised of 5 V-type immunoglobulin-like domains (D1-5) with 5-7 glycan chains, which increase the chains resistance to proteases. These glycosylation sites are located on one side of the protein and do not interfere with IgA binding. A long (10 amino acids) linker region exists between D3 and D4, so the D4 and D5 regions fold in on D2 and D3 in a compact J-shape. D1-3 are 12nm in length, while D4-5 are 10 nm long. Thus, D1 remains accessible. The one-sided glycans allow free access of D1's CDR regions and the Cys 502 at D5 to interact with IgA. It is thought that when D1 interacts with IgA's Fc region and the J chain, allowing the secretory component to unfold and disulfide formation between D5 C502 and IgA's Ch2 C311. While SC unfolds upon IgA binding, this binding imparts no change on the structure of IgA [3].
sIgA1 and sIgA2
Image:SIgA.jpg Adapted from Bonner, et al 2009 and Bonner, et al 2008. - Binding of the secretory component to the convex edge of the Fc region of dimeric IgA1 maintains in a near planar conformation, [5], [8]. The Fc regions align end to end without overlap, and the fab fragments remain in alignment with the Fc plane. In contrast, fab fragments remain out of alignment with the Fc plane. Because the secretory component resides at the convex region of the Fc portion, the D1 and D5 impart steric hindrance on the fab fragments, which are forced out of alignment. Consequently, IgA2 assumes a nonplanar conformation. The longer hinge region of IgA1 allows it to maintain its planar conformation.
Insights into Function
Structure and the Mucosal Environment
- Glycosylation of the IgA, the J chain, and the secretory component lends to protection against proteolytic attack in the harsh mucosal environment. Dimerization allows transcytosis. In addition, glycosylation on the secretory chain is thought to assist with anchoring to the mucosa. Through steric hindrance, the secretory component assists in preventing the binding of microorganisms to gut mucosa, impeding their entry into the mucosa [4], [9].
- The Fc portion is more susceptible to intestinal proteases than other regions of the IgA. This region of increased susceptibility is the precise region to which the secretory component remains after the pIgR is cleaved [1]. So, the secretory component offers the antibody additional protection against proteolytic cleavage. Binding to Fc region reduces flexibility at the hinge and between the 2 Fc regions. The loss in flexibility correlates with a decrease in the likelihood that the IgA will be in the correct conformation for cleavage to occur [3] and prevents large bacterial matrix metalloproteases from cleaving the Fc and hinge regions. So, the secretory component and dimeric IgA synergize to create a protected protein fit for the harsh mucosal environment.
Limiting Effector Responses through Decreased FcαR Binding
- The FcαR binding sites are located one per heavy chain at each Ch2-Ch3 interface. Both domains contribute one binding site. So, the stoichiometry between monomeric IgA and the FcαR is [2]. The Fc portion is shown in red, and the receptor is in blue. Dimerization would increase this stoichiometry 4:1; however, 2 of the binding sites will be . Because of constraints, only 1 of the 2 remaining binding sites will be available to bind receptor. Therefore, physiologic stoichiometry is 1:1.
- The binding of IgA to the FcαR does not elicit a structural change in the antibody [9]. Effector function is elicited when multiple receptors bind and resultant clustering triggers signaling events. The 1:1 stoichiometry greatly limits FcαR clustering and consequent effector functions by effectively limiting the concentration of available antibody binding sites in the local environment, favoring neutralization in the absence of cytotoxic and inflammatory responses upon antigen recognition. Additional modulation occurs through internal signaling events. For example, cytokines trigger changes in cytoskeletal arrangements that result in clustering of the FcαR at the cell surface. In effect, the interaction of the secretory component limits the effector and inflammatory responses upon antigen binding without limiting the ability of the antibody to neutralize pathogens or exclude commensals from breeching the mucosal barrier.
Differences in Antigen Binding
- While both IgA1 and IgA2 are able to bind polysaccharide, IgA1 preferentially binds protein antigen, while IgA2 preferentially binds lipopolysaccharide lipid A [5]. This difference in structure can be explained, at least in part, by structural differences. The binding of the secretory component to IgA1 results in a planar antibody with a wide, rigid antigenic reach. In contrast, secretory component binding to IgA2 results in a compact nonplanar form.
- Since is planar and more flexible, this might lend to antigen binding on proteins, which are larger and more variable [5]. Flexibility allows IgA1 access to a more diverse array of orientations. Likewise the more compact, nonplanar might preferentially bind repeating patterns on fixed surfaces, like bacteria coating intestinal mucosa. It is interesting to note that IgA2 tends to induce signaling more slowly than IgA1 upon binding FcαR. So, differences in isoform structure correspond to different antigen specificities and consequent differences in the roles each isoform plays in eliciting mucosal immune responses.
Conclusions on Function
- The secretory component interacts with either dimeric IgA1 or IgA2 to form a functional unit, structurally adapted to the harsh mucosal environment and to control potentially pathogenic mucosal flora primarily through neutralization. Inflammation is controlled by limiting the available binding sites on the Fc portion of IgA, effectively preventing FcαR clustering through a 1:1 stoichiometric binding. Differences in structure and resulting function allows the two isoforms fill unique niches in mucosal immune responses, suggesting selective advantages for each. Whereas IgA1 specializes in protein detection, IgA2 tends to bind LPS and polysaccharide antigen. So, structure arms the IgA secretory unit with specific advantages suited for its environmental, maintains balance between inflammation and mucosal barrier protection by limiting effector responses, and imparts unique functional roles to IgA isoforms. Together, structure and function determine the immune niches filled by IgA1 and IgA2.
Implications in Medicine and Science
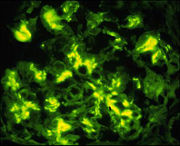 Immunofluorescence detecting IgA in IgA glomerulonephritis. From  , with permission - IgA nephropathy is the most prevalent cause of chronic glomerulonephritis in the world and is caused by polymeric IgA1 deposited at the kidney glomeruli [8]. Notably, 90% of serum IgA is IgA1, mostly in the monomeric form. The observation that individuals with IgA myeloma [1] lack nephropathy suggests an abnormality in IgA structure, leading to an abnormal amount of polymerization. Steric hindrance of the fab segments normally limits the amount of polymerization of IgA. Bonner, et al proposes that a disturbance in the hinge region or an absence of fab. Similarly, decreased O-glycosylation might could destabilize the hinge region, allowing IgA to self associate. Likewise, destabilizing this region might make IgA susceptible to cleavage of fab fragments by bacterial proteases, leading to self aggregation and renal pathology. For more information on IgA nephropathy: [2]. [10].
- In other areas of science, studying mouse models of pathologies involving IgA1 introduces an added variable since IgA1 is found in higher apes only [4]. Such complications in the experimental model must be taken into account when interpreting results.
Limitations of the Current Studies
- Because IgA has a high amount of glycosylation and a relatively large amount of flexibility, it has proven particularly difficult to crystallize in its intact form. Similarly, glycosylation and long linker regions between domains poses challenges to the crystallization of the secretory component. Alternative techniques employed in these studies included x-ray, neutron scattering analysis, analytical ultracentrifugation, and constrained modeling. Details provided in crystallographic studies – like disulfide bond, glycosylation residues and sites, detailed visualization of binding interaction – are absent in these results. Because of the limiting resolution of these models, many details concerning the binding residues and residue interactions are left unknown. Therefore, numerous questions are left unanswered, some of which are listed below. [8], [4], [2], [1]
Questions Unanswered (a few of many)
- What secretory component amino acids interact with the J chain?
- What CDR-like motifs of secretory component’s D1 bind, and where does this binding occur on IgA?
- What residues on the secretory component are glycosylated?
- What binding differences characterize IgA1 vs IgA2? [1]
- Why does IgA2 lack as robust an effector function in binding to FcαR?
- What are the precise binding motifs of the secretory component and IgA1? [8]
- What is the structure of IgA involved in IgA nephropathy? [8]
- Crystallographic structure will yield further insights into the structure of IgA, the interactions between IgA and other molecules.
Links
IgA
- Refined crystal structure of the galactan-binding immunoglobulin fab j539 at 1.95-angstroms resolution 2fbj
- Phosphocholine binding immunoglobulin fab mc/pc603. an x-ray diffraction study at 2.7 angstroms 1mcp
- Phosphocholine binding immunoglobulin fab mc/pc603. an x-ray diffraction study at 3.1 angstroms 2mcp
- Crystal structure of human FcaRI bound to IgA1-Fc 1ow0
- Refined crystal structure of a recombinant immunoglobulin domain and a complementarity-determining region 1-grafted mutant 2imm and2imn
- Crystal structure of a Staphylococcus aureus protein (SSL7) in complex with Fc of human IgA1 2qej
- Model of human IgA1 determined by solution scattering, curve-fitting, and homology modeling 1iga
- Model of human IgA2 determined by solution scattering, curve fitting and homology modelling 1r70
- Solution structure of human dimeric immunoglobulin A 2qtj
- Solution structure of human secretory IgA1 3chn
- Solution Structure of Human SIgA2 3cm9
- Solution structure of human secretory component 2ocw
Related Molecules
- non-IgA antibody isotypes
- IgM: Solution structure of human Immunoglobulin M 2rcj
- IgG: Crystal structure of the intact human IgG B12 with broad and potent activity against primary HIV-1 isolates: a template for HIV vaccine design 1hzh
- IgG: Three=dimensional structure of a human immunoglobulin with a hinge deletion 1mco
- IgD: Semi-extended solution structure of human myeloma immunoglobulin D determined by constrained X-ray scattering 1zvo
- IgE: Structure of the human ige-fc bound to its high affinity receptor fc(epsilon)ri(alpha) 1f6a
- Other C-type immunoglobulin examples
- MHC: Crystal Structure of monomeric human beta-2-microglobulin 1lds
- TCR: Crystal Structure of the G17E/A52V/S54N/Q72H/E80V/L81S/T87S/G96V variant of the murine T cell receptor V beta 8.2 domain 2apv
- V-type immunoglobulin examples
- Crystal Structure of a Ligand-Binding Domain of the Human Polymeric Ig Receptor, pIgR 1xed
- Crystal structure of human FcaRI 1ovz
- Influenza virus hemagglutinin complexed with a neutralizing antibody 1qfu
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Bonner A, Perrier C, Corthesy B, Perkins SJ. Solution structure of human secretory component and implications for biological function. J Biol Chem. 2007 Jun 8;282(23):16969-80. Epub 2007 Apr 11. PMID:17428798 doi:http://dx.doi.org/10.1074/jbc.M701281200
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Furtado PB, Whitty PW, Robertson A, Eaton JT, Almogren A, Kerr MA, Woof JM, Perkins SJ. Solution structure determination of monomeric human IgA2 by X-ray and neutron scattering, analytical ultracentrifugation and constrained modelling: a comparison with monomeric human IgA1. J Mol Biol. 2004 May 14;338(5):921-41. PMID:15111057 doi:http://dx.doi.org/10.1016/j.jmb.2004.03.007
- ↑ 3.0 3.1 3.2 3.3 3.4 Bonner A, Almogren A, Furtado PB, Kerr MA, Perkins SJ. Location of secretory component on the Fc edge of dimeric IgA1 reveals insight into the role of secretory IgA1 in mucosal immunity. Mucosal Immunol. 2009 Jan;2(1):74-84. Epub 2008 Oct 8. PMID:19079336 doi:http://dx.doi.org/10.1038/mi.2008.68
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Boehm MK, Woof JM, Kerr MA, Perkins SJ. The Fab and Fc fragments of IgA1 exhibit a different arrangement from that in IgG: a study by X-ray and neutron solution scattering and homology modelling. J Mol Biol. 1999 Mar 12;286(5):1421-47. PMID:10064707 doi:http://dx.doi.org/10.1006/jmbi.1998.2556
- ↑ 5.0 5.1 5.2 5.3 Bonner A, Almogren A, Furtado PB, Kerr MA, Perkins SJ. The nonplanar secretory IgA2 and near planar secretory IgA1 solution structures rationalize their different mucosal immune responses. J Biol Chem. 2009 Feb 20;284(8):5077-87. Epub 2008 Dec 23. PMID:19109255 doi:http://dx.doi.org/10.1074/jbc.M807529200
- ↑ 6.0 6.1 6.2 6.3 Attwood, T. "Immunoglobulin superfamily " ImPrints Retrieved April, 2009, from http://www.jenner.ac.uk/Bioinformatics/ImPRINTS/immunoglobulin_superfamily_background.htm.
- ↑ (nov 22 2007). "Superfamily: immunoglobulin." SCOP, from http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.c.b.b.html.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Bonner A, Furtado PB, Almogren A, Kerr MA, Perkins SJ. Implications of the near-planar solution structure of human myeloma dimeric IgA1 for mucosal immunity and IgA nephropathy. J Immunol. 2008 Jan 15;180(2):1008-18. PMID:18178841
- ↑ 9.0 9.1 Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature. 2003 Jun 5;423(6940):614-20. Epub 2003 May 21. PMID:12768205 doi:http://dx.doi.org/10.1038/nature01685
- ↑ Falk, R. "IgA Nephropathy." UNC Kidney Center, from http://www.unckidneycenter.org/kidneyhealthlibrary/iganephropathy.html.
--Rebecca Martin 01:23, 2 May 2009 (IDT)
|
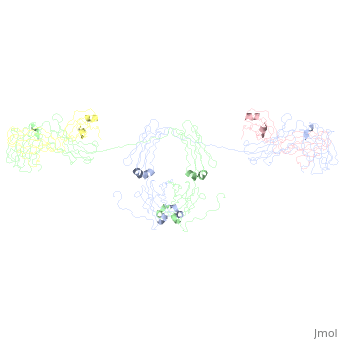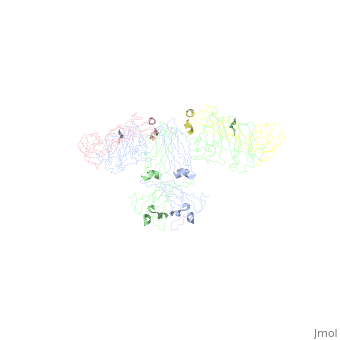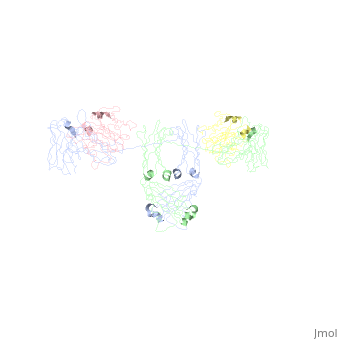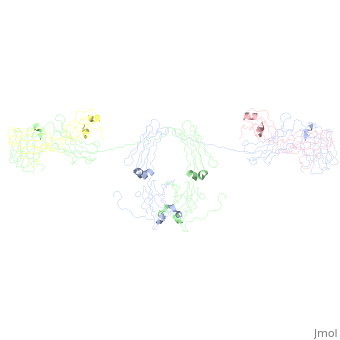

 , with permission
, with permission