We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Prp40
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
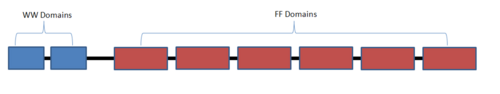
==The first FF domain (FF1)== | ==The first FF domain (FF1)== | ||
| - | |||
| - | |||
| - | <Structure load='1o6w' size='250' thumb='false' align='left' caption='Figure 2: N terminal (blue) to C terminal (red) ribbon representation of the consecutive WW domains of Prp40, [[1o6w]]' scene='Sandbox_504/Start_scene_ff1/1'/> | ||
| - | |||
The <scene name='Sandbox_504/Start_scene_ff1/1'>FF1 domain of Prp40 is comprised of three alpha helices</scene>, and one 310 helix located between α2 and α3. Helices are composed of the following residues, <scene name='Sandbox_504/Ff1_a1/1'>α1</scene> (134-146), <scene name='Sandbox_504/Ff1_a2/2'>α2</scene> (154-163), <scene name='Sandbox_504/Ff1_310/1'>310</scene> (167-170), and <scene name='Sandbox_504/Ff1_a3/1'>α3</scene> (175-187). The core domain is made up of a series of aromatic and aliphatic residues. A type 1 β-turn is exhibited by the residues Asp149, Ser150, Thr151, and Trp152<ref name ="gasch"/>. | The <scene name='Sandbox_504/Start_scene_ff1/1'>FF1 domain of Prp40 is comprised of three alpha helices</scene>, and one 310 helix located between α2 and α3. Helices are composed of the following residues, <scene name='Sandbox_504/Ff1_a1/1'>α1</scene> (134-146), <scene name='Sandbox_504/Ff1_a2/2'>α2</scene> (154-163), <scene name='Sandbox_504/Ff1_310/1'>310</scene> (167-170), and <scene name='Sandbox_504/Ff1_a3/1'>α3</scene> (175-187). The core domain is made up of a series of aromatic and aliphatic residues. A type 1 β-turn is exhibited by the residues Asp149, Ser150, Thr151, and Trp152<ref name ="gasch"/>. | ||
==The fourth FF domain (FF4)== | ==The fourth FF domain (FF4)== | ||
| - | + | <scene name='Sandbox_504/Start_scene_ff4/1'>Domain FF4 of Prp40</scene> exhibits compact four helical bundle fold comprised of an α1-α2-310-α3 topology. The composition of each helix is a follows <scene name='Sandbox_504/A1_of_ff4/1'>α1</scene> (Glu489-Thr507), <scene name='Sandbox_504/A2_of_ff4/1'>α2</scene> (Trp519-Leu526), <scene name='Sandbox_504/310_of_ff4/1'>310</scene> (Tyr532-Gly536) and <scene name='Sandbox_504/A3_of_ff4/1'>α3</scene> (Asp539-Phe549). There are a series of interactions between the different helices, for example Tyr532 is in contact with Phe500, Leu503, Ser523, and Arg542. A difference between FF1 and FF4 is the presence of five extra amino acids in F4 which gives the <scene name='Sandbox_504/Loop_of_ff4/1'>loop</scene> located between α 1 and α2 an extra turn. This insertion however does not increase the flexibility of F4 as compared to F1<ref name ="bonet"/>. | |
| - | + | </StructureSection> | |
| - | < | + | |
| - | + | ||
| - | Domain FF4 of Prp40 exhibits compact four helical bundle fold comprised of an α1-α2-310-α3 topology. The composition of each helix is a follows <scene name='Sandbox_504/A1_of_ff4/1'>α1</scene> (Glu489-Thr507), <scene name='Sandbox_504/A2_of_ff4/1'>α2</scene> (Trp519-Leu526), <scene name='Sandbox_504/310_of_ff4/1'>310</scene> (Tyr532-Gly536) and <scene name='Sandbox_504/A3_of_ff4/1'>α3</scene> (Asp539-Phe549). There are a series of interactions between the different helices, for example Tyr532 is in contact with Phe500, Leu503, Ser523, and Arg542. A difference between FF1 and FF4 is the presence of five extra amino acids in F4 which gives the <scene name='Sandbox_504/Loop_of_ff4/1'>loop</scene> located between α 1 and α2 an extra turn. This insertion however does not increase the flexibility of F4 as compared to F1<ref name ="bonet"/>. | + | |
| - | + | ||
=Additional Resources= | =Additional Resources= | ||
*[http://www.rcsb.org/pdb/explore/explore.do?structureId=2KFD Prp40 FF4 domain, in the RCSB Protein Data Bank] | *[http://www.rcsb.org/pdb/explore/explore.do?structureId=2KFD Prp40 FF4 domain, in the RCSB Protein Data Bank] | ||
Revision as of 09:17, 8 April 2013
| |||||||||||
Additional Resources
- Prp40 FF4 domain, in the RCSB Protein Data Bank
- SOLUTION STRUCTURE OF THE PRP40 WW DOMAIN PAIR OF THE YEAST SPLICING FACTOR PRP40, in the RCSB Protein Data Bank
- First FF domain of Prp40 Yeast Protein, in the RCSB Protein Data Bank
References
- ↑ 1.0 1.1 Bonet R, Ruiz L, Morales B, Macias MJ. Solution structure of the fourth FF domain of yeast Prp40 splicing factor. Proteins. 2009 Dec;77(4):1000-3. PMID:19722265 doi:10.1002/prot.22547
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Wiesner S, Stier G, Sattler M, Macias MJ. Solution structure and ligand recognition of the WW domain pair of the yeast splicing factor Prp40. J Mol Biol. 2002 Dec 6;324(4):807-22. PMID:12460579
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Gasch A, Wiesner S, Martin-Malpartida P, Ramirez-Espain X, Ruiz L, Macias MJ. The structure of Prp40 FF1 domain and its interaction with the crn-TPR1 motif of Clf1 gives a new insight into the binding mode of FF domains. J Biol Chem. 2006 Jan 6;281(1):356-64. Epub 2005 Oct 27. PMID:16253993 doi:10.1074/jbc.M508047200
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 van der Feltz C, Anthony K, Brilot A, Pomeranz Krummel DA. Architecture of the Spliceosome. Biochemistry. 2012 Apr 10. PMID:22471593 doi:10.1021/bi201215r
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Sperling J, Azubel M, Sperling R. Structure and function of the Pre-mRNA splicing machine. Structure. 2008 Nov 12;16(11):1605-15. PMID:19000813 doi:10.1016/j.str.2008.08.011
- ↑ Zhang L, Xu T, Maeder C, Bud LO, Shanks J, Nix J, Guthrie C, Pleiss JA, Zhao R. Structural evidence for consecutive Hel308-like modules in the spliceosomal ATPase Brr2. Nat Struct Mol Biol. 2009 Jul;16(7):731-9. Epub 2009 Jun 14. PMID:19525970 doi:10.1038/nsmb.1625
- ↑ Zhang L, Xu T, Maeder C, Bud LO, Shanks J, Nix J, Guthrie C, Pleiss JA, Zhao R. Structural evidence for consecutive Hel308-like modules in the spliceosomal ATPase Brr2. Nat Struct Mol Biol. 2009 Jul;16(7):731-9. Epub 2009 Jun 14. PMID:19525970 doi:10.1038/nsmb.1625
- ↑ 8.0 8.1 Cramer P, Bushnell DA, Kornberg RD. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science. 2001 Jun 8;292(5523):1863-76. Epub 2001 Apr 19. PMID:11313498 doi:http://dx.doi.org/10.1126/science.1059493
- ↑ Phatnani HP, Greenleaf AL. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 2006 Nov 1;20(21):2922-36. PMID:17079683 doi:10.1101/gad.1477006
- ↑ 10.0 10.1 10.2 10.3 Morris DP, Greenleaf AL. The splicing factor, Prp40, binds the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem. 2000 Dec 22;275(51):39935-43. PMID:10978320 doi:10.1074/jbc.M004118200