Molecular Playground/PcrA Helicase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<StructureSection load='1pjr' size='450' side='right' scene='' caption=''> | <StructureSection load='1pjr' size='450' side='right' scene='' caption=''> | ||
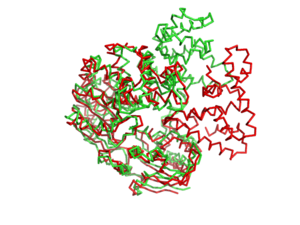
| - | [[Image:1jprto3jpr.png|left|300px]] '''Helicases''' are nucleic acid–dependent ATP-ases that are capable of unwinding [[DNA]] or RNA duplex substrates. As a consequence, they play roles in almost every process in cells that involves nucleic acids, including DNA replication and repair, transcription, translation, ribosome synthesis (1). See: <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_1/1pjrglobular/1'>Space fill Model</scene><br/> | + | [[Image:1jprto3jpr.png|left|300px]] '''Helicases''' are nucleic acid–dependent ATP-ases that are capable of unwinding [[DNA]] or RNA duplex substrates. As a consequence, they play roles in almost every process in cells that involves nucleic acids, including DNA replication and repair, transcription, translation, ribosome synthesis (1). See: <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_1/1pjrglobular/1'>Space fill Model</scene><br/> (PDB entry [[1pjr]]). |
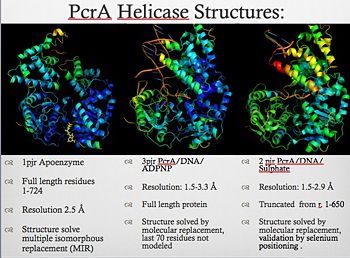
==PcrA a Simple Model for Helicases== | ==PcrA a Simple Model for Helicases== | ||
| Line 10: | Line 10: | ||
==PcrA Biochemistry== | ==PcrA Biochemistry== | ||
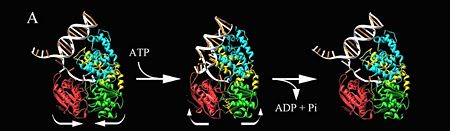
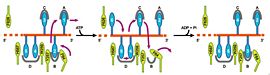
| - | [[Image:Wigleypcr4.jpg|left|450px]] PcrA is has an ATPas activityt which directionality is from 3' to 5' helicase strand separation reaction. The enzyme shows a specificity for the DNA substrate in gel mobility assays with the preferred substrate being one containing both single and double stranded regions of DNA. In contrast to Rep and UvrD from E. coli, there is not evidence for dimerisation of the enzyme using gel filtration, or by crosslinking in the presence of combinations of Mg2+, nucleotides and DNA. Moreover, kcat for ATP hydrolysis is constant over a large range of protein concentrations. Therefore, the protein appears to be monomeric under all conditions tested, including in the structure of two crystal forms of PcrA.[http://www.icnet.uk/labs/wigley/projects/helicase/35.html]<br/> | + | [[Image:Wigleypcr4.jpg|left|450px]] |
| + | {{clear}} | ||
| + | PcrA is has an ATPas activityt which directionality is from 3' to 5' helicase strand separation reaction. The enzyme shows a specificity for the DNA substrate in gel mobility assays with the preferred substrate being one containing both single and double stranded regions of DNA. In contrast to Rep and UvrD from E. coli, there is not evidence for dimerisation of the enzyme using gel filtration, or by crosslinking in the presence of combinations of Mg2+, nucleotides and DNA. Moreover, kcat for ATP hydrolysis is constant over a large range of protein concentrations. Therefore, the protein appears to be monomeric under all conditions tested, including in the structure of two crystal forms of PcrA.[http://www.icnet.uk/labs/wigley/projects/helicase/35.html]<br/> | ||
<br/> | <br/> | ||
Revision as of 09:31, 10 April 2013
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Alexander Berchansky, Luis E Ramirez-Tapia