Molecular Playground/PcrA Helicase
From Proteopedia
| Line 17: | Line 17: | ||
<br/> | <br/> | ||
| - | ==PcrA Helicase Mechanism : The Mexican Wave== | + | ==PcrA Helicase Mechanism: The Mexican Wave== |
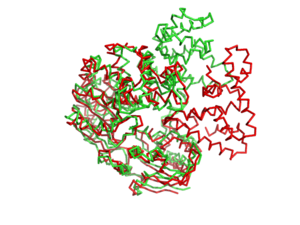
Professor Dale B. Wigley' group in 1996-1999 was able to crystalize the intermediate states from PcrA, giving solution to the controversy of what kind of mechanism this helicase has. [http://www.ncbi.nlm.nih.gov/pubmed/10199404ordinalpos=39&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum] | Professor Dale B. Wigley' group in 1996-1999 was able to crystalize the intermediate states from PcrA, giving solution to the controversy of what kind of mechanism this helicase has. [http://www.ncbi.nlm.nih.gov/pubmed/10199404ordinalpos=39&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum] | ||
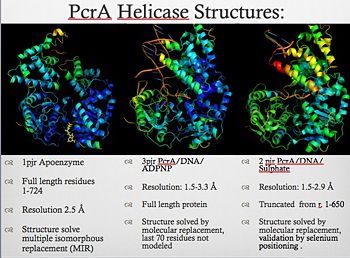
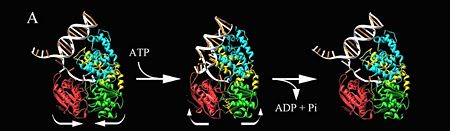
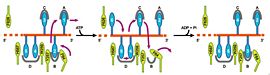
| - | Two crystal form of the enzyme, one couple with a 10 mer DNA and a non hydrolizable form of ATP (ATPnP)([[3pjr]]), <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_2/3pjrinitial/1'>(Enzyme Subtrate Structure)</scene> and another a truncated form embebed in sulfate ([[2pjr]])<scene name='User:Luis_E_Ramirez-Tapia/Sandbox_2/2pjrinitial/1'>(Enzyme Product Structure)</scene>, give a light in a model for how ATP hydrolysis results in motor movement along ssDNA. In the figure below step 1 (top) is the ATP free (product) ssDNA conformation. The DNA bases are labelled arbitrarily. On binding ATP, F626 creates a new binding pocket for base 6. Likewise, F64 destroys an acceptor pocket for base 2, forcing it to move to the position occupied by base 1. After ATP hydrolysis, the grip on base 6 is released. When the Y257 pocket is re-opened due to movement of F64, bases 3-6 can now flip through the acceptor pockets to their new positions. This model predicts that each ATP hydrolysis event will advance PcrA one base along ssDNA.[http://www.icnet.uk/labs/wigley/projects/helicase/35.html] | + | Two crystal form of the enzyme, one couple with a 10 mer DNA and a non hydrolizable form of ATP (ATPnP)([[3pjr]]), <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_2/3pjrinitial/1'>(Enzyme Subtrate Structure)</scene> and another a truncated form embebed in sulfate ([[2pjr]]) <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_2/2pjrinitial/1'>(Enzyme Product Structure)</scene>, give a light in a model for how ATP hydrolysis results in motor movement along ssDNA. In the figure below step 1 (top) is the ATP free (product) ssDNA conformation. The DNA bases are labelled arbitrarily. On binding ATP, F626 creates a new binding pocket for base 6. Likewise, F64 destroys an acceptor pocket for base 2, forcing it to move to the position occupied by base 1. After ATP hydrolysis, the grip on base 6 is released. When the Y257 pocket is re-opened due to movement of F64, bases 3-6 can now flip through the acceptor pockets to their new positions. This model predicts that each ATP hydrolysis event will advance PcrA one base along ssDNA.[http://www.icnet.uk/labs/wigley/projects/helicase/35.html] |
[[Image:Mexicanwave.jpg|thumb|270px|left|Inchworm or Mexicanwave model]] '''The link below shows a movie with the principal characteristics of this protein as long with the inchworm model'''. [http://www.youtube.com/watch?v=veY0LlL7Dt0 Pcr4 Helicase and Mexican Wave] | [[Image:Mexicanwave.jpg|thumb|270px|left|Inchworm or Mexicanwave model]] '''The link below shows a movie with the principal characteristics of this protein as long with the inchworm model'''. [http://www.youtube.com/watch?v=veY0LlL7Dt0 Pcr4 Helicase and Mexican Wave] | ||
{{clear}} | {{clear}} | ||
| Line 39: | Line 39: | ||
For additional information, see: [[Bacterial Infections]] | For additional information, see: [[Bacterial Infections]] | ||
<br /> | <br /> | ||
| - | + | </StructureSection> | |
| - | == | + | ==References== |
Crystal structure of a DExx box DNA helicase., Subramanya HS, Bird LE, Brannigan JA, Wigley DB, Nature. 1996 Nov 28;384(6607):379-83. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8934527 8934527] | Crystal structure of a DExx box DNA helicase., Subramanya HS, Bird LE, Brannigan JA, Wigley DB, Nature. 1996 Nov 28;384(6607):379-83. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8934527 8934527] | ||
[[Category: Geobacillus stearothermophilus]] | [[Category: Geobacillus stearothermophilus]] | ||
| Line 55: | Line 55: | ||
[[Category: Sos response]] | [[Category: Sos response]] | ||
| - | + | ||
^ Johnson DS, Bai L, Smith BY, Patel SS, Wang MD (2007). "Single-molecule studies reveal dynamics of DNA unwinding by the ring-shaped t7 helicase". Cell 129 (7): 1299–309. doi:10.1016/j.cell.2007.04.038. PMID 17604719. | ^ Johnson DS, Bai L, Smith BY, Patel SS, Wang MD (2007). "Single-molecule studies reveal dynamics of DNA unwinding by the ring-shaped t7 helicase". Cell 129 (7): 1299–309. doi:10.1016/j.cell.2007.04.038. PMID 17604719. | ||
Revision as of 09:52, 10 April 2013
| |||||||||||
References
Crystal structure of a DExx box DNA helicase., Subramanya HS, Bird LE, Brannigan JA, Wigley DB, Nature. 1996 Nov 28;384(6607):379-83. PMID:8934527
^ Johnson DS, Bai L, Smith BY, Patel SS, Wang MD (2007). "Single-molecule studies reveal dynamics of DNA unwinding by the ring-shaped t7 helicase". Cell 129 (7): 1299–309. doi:10.1016/j.cell.2007.04.038. PMID 17604719. ^ a b "Researchers solve mystery of how DNA strands separate" (2007-07-03). Retrieved on 2007-07-05. ^ Dumont S, Cheng W, Serebrov V, Beran RK, Tinoco Jr I, Pylr AM, Bustamante C, "RNA Translocation and Unwinding Mechanism of HCV NS3 Helicase and its Coordination by ATP", Nature. 2006 Jan 5; 439: 105-108. Anand SP, Zheng H, Bianco PR, Leuba SH, Khan SA. DNA helicase activity of PcrA is not required for displacement of RecA protein from DNA or inhibition of RecA-mediated DNA strand exchange. Journal of Bacteriology (2007) 189 (12):4502-4509. Bird L, Subramanya HS, Wigley DB, "Helicases: a unifying structural theme?", Current Opinion in Structural Biology. 1998 Feb; 8 (1): 14-18. Betterton MD, Julicher F, "Opening of nucleic-acid double strands by helicases: active versus passive opening.", Physical Review E. 2005 Jan; 71 (1): 011904.
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Alexander Berchansky, Luis E Ramirez-Tapia