This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Michael Roberts/BIOL115 Chymo
From Proteopedia
| Line 11: | Line 11: | ||
Click on the ''' 'green links' ''' in the text in the scrollable section below to examine this molecule in more detail. | Click on the ''' 'green links' ''' in the text in the scrollable section below to examine this molecule in more detail. | ||
| - | <StructureSection load='1afq' size=' | + | <StructureSection load='1afq' size='700' side='right' caption='Structure of bovine chymotrypsin (PDB entry [[1afq]])' scene='User:Michael_Roberts/BIOL115_Chymo/Start/1'> |
== Tertiary structure == | == Tertiary structure == | ||
Chymotrypsin is initially synthesized as a 245 amino acid inactive precursor (a zymogen) termed chymotrypsinogen. Activation of chymotrypsinogen involves proteolytic cleavage at two sites along the chain and removal of two amino acids at each cleavage site. The resultant <scene name='User:Michael_Roberts/BIOL115_Chymo/Chains/1'>three chains</scene> are shown here (chain 1 = 1-13 in green; chain 2 = 16-146 in red; chain 3 = 149-24 in blue). Note, some amino acids at the temini of these chains are not shown in this representation (e.g. 11-13, 149, ). This is because these residues show too much flexibility in the crystal structures to give X-ray diffraction patterns which would locate them in space. | Chymotrypsin is initially synthesized as a 245 amino acid inactive precursor (a zymogen) termed chymotrypsinogen. Activation of chymotrypsinogen involves proteolytic cleavage at two sites along the chain and removal of two amino acids at each cleavage site. The resultant <scene name='User:Michael_Roberts/BIOL115_Chymo/Chains/1'>three chains</scene> are shown here (chain 1 = 1-13 in green; chain 2 = 16-146 in red; chain 3 = 149-24 in blue). Note, some amino acids at the temini of these chains are not shown in this representation (e.g. 11-13, 149, ). This is because these residues show too much flexibility in the crystal structures to give X-ray diffraction patterns which would locate them in space. | ||
Revision as of 16:26, 1 May 2013
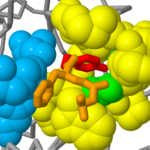
Chymotrypsin.
Chymotrypsin is a member of a family of enzymes all of which cleave peptide bonds through the action of an active site serine (the serine proteases).
This family includes the pancreatic enzymes chymotrypsin, trypsin and elastase as well as a variety of other proteases (e.g. cocoonase, thrombin, acrosomal protease, etc.). Chymotrypsin, trypsin and elastase show a high degree of similarity in their overall tertiary structure, but have different substrate specificities determined by the different properties of the substrate binding site on each enzyme.
Click on the 'green links' in the text in the scrollable section below to examine this molecule in more detail.
| |||||||||||
