User:Michael Roberts/BIOL115 CaM
From Proteopedia
| Line 56: | Line 56: | ||
'''CALMODULIN INTERACTS WITH ITS TARGET:''' | '''CALMODULIN INTERACTS WITH ITS TARGET:''' | ||
| - | The Ca<sup>2+</sup>-bound form of calmodulin with its exposed hydrophobic surfaces that you have already observed can <scene name='User:Michael_Roberts/BIOL115_CaM/Active_calmodulin/1'>interact with a target protein</scene>. It does this by wrapping around a specific sequence on the target molecule, | + | The Ca<sup>2+</sup>-bound form of calmodulin with its exposed hydrophobic surfaces that you have already observed can <scene name='User:Michael_Roberts/BIOL115_CaM/Active_calmodulin/1'>interact with a target protein</scene>. It does this by wrapping around a specific sequence on the target molecule, which is then forced into an α-helical structure. |
The target molecule here (shown in blue) is the calmodulin-regulated enzyme, myosin light chain kinase. Only a short sequence from this protein, the calmodulin binding domain, is shown. | The target molecule here (shown in blue) is the calmodulin-regulated enzyme, myosin light chain kinase. Only a short sequence from this protein, the calmodulin binding domain, is shown. | ||
Revision as of 16:28, 3 May 2013
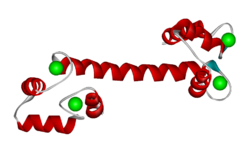
Sequence and structure of EF hands
The EF hand motif is present in a many proteins and it commonly bestows the ability to bind Ca2+ ions. It was first identified in parvalbumin, a muscle protein. Here we'll have a look at the Ca2+-binding protein calmodulin, which possesses four EF hands. Calmodulin and its isoform, troponinC, are important intracellular Ca2+-binding proteins.
The structure below, obtained by X-ray crystallography, represents the Ca2+-binding protein calmodulin. It has a dumbell-shaped structure with two identical lobes connected by a central alpha-helix. Each lobe comprises three α-helices joined by loops. A helix-loop-helix motif forms the basis of each EF hand.
Click on the 'green links' in the text in the scrollable section below to examine this molecule in more detail.
| |||||||||||
External Resources. You can view a nice animation of the conformational change undergone by calmodulin upon calcium binding by following this link [1].
