Ricin
From Proteopedia
| Line 1: | Line 1: | ||
| - | + | '''Ricin''' is a potent cytotoxin that is synthesized in the endosperm cells of maturing seeds of the castor oil plant (''Ricinus communis'')<ref name="lord">PMID: 8119491</ref>. Ricin belongs to a small multi-gene family<ref name="montfort">PMID: 3558397</ref> that is composed of eight members. Ricin is classified as a type II heterodimeric Ribosome Inactivating Protein<ref name="lord" /> or RIPs. For toxins in Proteopedia see [[Toxins]]. | |
| + | <StructureSection load='3rtj' size='400' side='right' caption='Ricin bound to adenine (PDB entry [[3rtj]])'> | ||
| + | __notoc__ | ||
| + | ==Structure== | ||
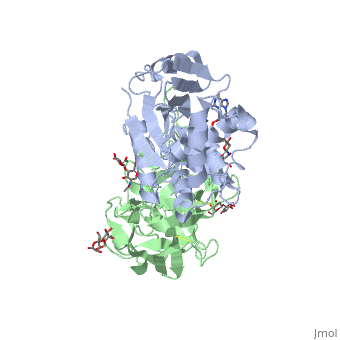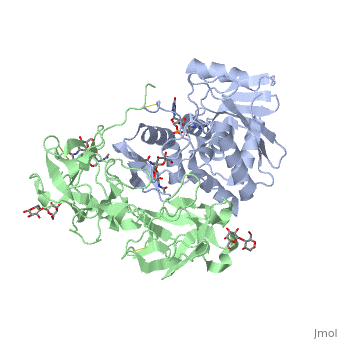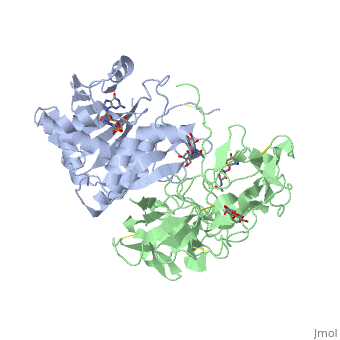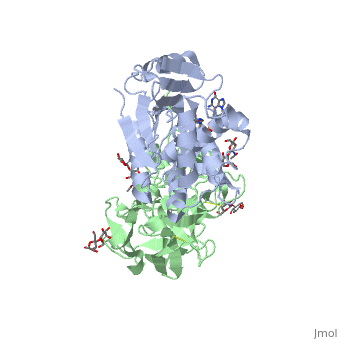
| + | Ricin is a heterodimer that consists of a 32 kilodalton A chain glycoprotein (light blue) linked by a <scene name='Sandbox_BCMB402_Ricin/Dihedral_link_between_subunits/1'>disulfide bond</scene> to a 32 kilodalton <scene name='Sandbox_BCMB402_Ricin/B_subunit/1'>B chain</scene> glycoprotein<ref name="montfort" /> (green). | ||
| - | = | + | The <scene name='Sandbox_BCMB402_Ricin/A_subunit_secondary_structure/2'> A chain</scene> is an alpha/beta protein which contains eight alpha helices (pink) and eight beta sheets (yellow). It has three domains<ref name="Weston">PMID: 7990130</ref>. <scene name='Sandbox_BCMB402_Ricin/Domain_1_of_a_subunit/2'>Domain 1 </scene> consists of a beta sheet containing both parallel and anti-parallel strands. The <scene name='Sandbox_BCMB402_Ricin/Domain2_of_a_subunit/1'> second alpha helical domain </scene> makes up the core of the protein, and includes the active site. The<scene name='Sandbox_BCMB402_Ricin/Domain3_of_a_subunit/1'> third domain</scene> interacts with the B chain, and contains a helix and two beta strands. |
| - | + | ||
| - | + | The A chain contains the active site that is responsible for inactivating the [[Ribosome]] via depurination. RIPs have very diverse structures, containing only eight invariant residues<ref name = "lord"/>. These <scene name='Sandbox_BCMB402_Ricin/Conserved_residues/2'>conserved residues</scene> are clustered in the active site. | |
| - | + | ||
| - | + | ||
| - | ==Physiology== | ||
| - | The mechanism deployed by Ricin to gain entry to a host cell involves the poison's heterogenic properties. First, the toxin arranges itself in such a way where its B chain can easily interact with the host cells receptors, and once acknowledgement happens, the B chain can facilitate transport of the A chain into the cytoplasm<ref name="montfort" />. This association between the A and B chain is essential for toxicity<ref name="montfort" /> without it the Ricin would not be able to gain access to the cells organelles rendering it useless. Once the A chain gains entry into the cytosol its mechanism for attack of the [[Ribosome|ribosome]] is depurination of a single adenosine residue in a highly conserved portion within the large RNA of the cytoplasmic [[Large Ribosomal Subunit of Haloarcula|large ribosomal subunit]]<ref name="rapak" /> of eukaryotes; in human, the large cytoplasmic ribosomal RNA is called the 28S ribosomal RNA because of its sedimentation properties during ultracentrifugation. The nucleotide depurinated is located within a specific, conserved loop referred to as th <nowiki>'</nowiki>sarcin-ricin loop<nowiki>'</nowiki>; the loop is critical for binding [[elongation factors|elongation factors]] during [[Translation|translation]] of messenger RNA to protein <ref name="holmbergnygard">PMID: 8648651</ref>. Depurination of the single adenosine nucleotide by the toxin results in the inhibition of protein synthesis. | ||
| - | == | + | The B chain is a lectin<ref name="lord" /> that <scene name='Sandbox_BCMB402_Ricin/Carbohydrate_binding/1'>binds</scene> to galactose-containing surface receptors. Originally it was thought that the mode of action of Ricin poisoning was due to hemagglutination due to a closely related, co-isolating lectin, RCA. |
| - | '' | + | __notoc__ |
| + | ==Mechanism of action== | ||
| + | The mechanism deployed by Ricin to gain entry to a host cell involves the poison's heterogenic properties. First, the B subunit binds to two carbohydrates on the cell surface, either glycolipids or glycoproteins, which both terminate with galactose. The interaction is facilitated by hydrogen bonds to <scene name='Sandbox_BCMB402_Ricin/B_chain_bind_lactose_1/2'>lysine 40 and asparagine 46</scene> in one domain<ref name = "Rutenber">PMID: 3561502</ref> and <scene name='Sandbox_BCMB402_Ricin/B_chain_bind_lactose_2/1'>asparagine 255</scene> in the other domain. Once bound, the ricin-glycoprotein complex is taken into the cells via endocytosis, and the toxin is released into the cells. This association between the A and B chain is essential for toxicity <ref name="montfort" /> without it the Ricin would not be able to gain access to the cell, rendering it useless<ref name = "rapak">PMID: 9108055</ref>.. | ||
| + | Once the A chain gains entry into the cytosol its mechanism for attack of the [[Ribosome|ribosome]] is depurination of a single adenosine residue in a highly conserved portion within the large RNA of the cytoplasmic [[Large Ribosomal Subunit of Haloarcula|large ribosomal subunit]]<ref name="rapak" /> of eukaryotes; in human, the large cytoplasmic ribosomal RNA is called the 28S ribosomal RNA because of its sedimentation properties during ultracentrifugation. The nucleotide depurinated is located within a specific, conserved loop referred to as the <nowiki>'</nowiki>sarcin-ricin loop<nowiki>'</nowiki>; the loop is critical for binding [[elongation factors|elongation factors]] during [[Translation|translation]] of messenger RNA to protein <ref name="holmbergnygard">PMID: 8648651</ref>. Depurination of the single adenosine nucleotide by the toxin results in the inhibition of protein synthesis. | ||
| + | |||
| + | The proposed mechanism of depurination utilizes the <scene name='Sandbox_BCMB402_Ricin/Conserved_residues/2'>conserved residues</scene> in the A chain. The aromatic ring structures of the substrate adenosine stack with the aromatic side chains of <scene name='Sandbox_BCMB402_Ricin/Tyr_stacking/1'>two tyrosine residues</scene>, Tyr 80 and 123, above and below. Hydrogen bonds form between the conserved arginine and a backbone carbonyl. The depurination reaction is aided by the protonation of N3 by Arg 180 and by ion pairing to Glu 177. A water molecule on the opposite side of the ribose is activated by hydrogen bonding to Arg 180. The activated water attacks C1' of the ribose, releasing the adenine and depurinated RNA fragment. This interferes with elongation factor binding to the ribosome, thus inhibiting [[translation|translation]]. | ||
| + | </StructureSection> | ||
| + | |||
| + | ''Updated April 2013'' | ||
| + | __notoc__ | ||
===Ricin A chain (RTA)=== | ===Ricin A chain (RTA)=== | ||
[[1j1m]], [[1ift]], [[2aai]], [[1rtc]] – RTA<br /> | [[1j1m]], [[1ift]], [[2aai]], [[1rtc]] – RTA<br /> | ||
[[3lc9]], [[3mk9]], [[2vc4]], [[1uq4]], [[1uq5]], [[1obs]], [[3bjg]], [[3srp]] – RTA (mutant) | [[3lc9]], [[3mk9]], [[2vc4]], [[1uq4]], [[1uq5]], [[1obs]], [[3bjg]], [[3srp]] – RTA (mutant) | ||
| - | + | __notoc__ | |
===Ricin A chain binary complexes=== | ===Ricin A chain binary complexes=== | ||
| Line 37: | Line 45: | ||
[[1obt]] - RTA (mutant) + AMP<br /> | [[1obt]] - RTA (mutant) + AMP<br /> | ||
[[1apg]] – RTA + RNA | [[1apg]] – RTA + RNA | ||
| - | + | [[3px8]] – RTA + formycin monophosphate<br /> | |
| + | __notoc__ | ||
===Ricin B chain (RTB)=== | ===Ricin B chain (RTB)=== | ||
| Line 43: | Line 52: | ||
[[3nbe]] – CnRTB + lactose derivative<br /> | [[3nbe]] – CnRTB + lactose derivative<br /> | ||
[[3phz]] – RTB + glycoside – ''Polyporus squamosus'' | [[3phz]] – RTB + glycoside – ''Polyporus squamosus'' | ||
| - | + | __notoc__ | |
===Ricin A+B chains=== | ===Ricin A+B chains=== | ||
| - | [[ | + | [[2aai]] - RTA + RTB |
| - | + | ||
| + | [[3rtj]] - RTA + RTB + dinucleotide | ||
| + | __notoc__ | ||
==See Also== | ==See Also== | ||
* [[Ribosome]] | * [[Ribosome]] | ||
* [[Large Ribosomal Subunit of Haloarcula|Large Ribosomal Subunit]] | * [[Large Ribosomal Subunit of Haloarcula|Large Ribosomal Subunit]] | ||
* [[Translation]] | * [[Translation]] | ||
| - | + | __notoc__ | |
==References== | ==References== | ||
{{Reflist}} | {{Reflist}} | ||
| - | |||
| - | [[Category:Topic Page]] | ||
Revision as of 19:39, 3 May 2013
Ricin is a potent cytotoxin that is synthesized in the endosperm cells of maturing seeds of the castor oil plant (Ricinus communis)[1]. Ricin belongs to a small multi-gene family[2] that is composed of eight members. Ricin is classified as a type II heterodimeric Ribosome Inactivating Protein[1] or RIPs. For toxins in Proteopedia see Toxins.
| |||||||||||
Updated April 2013
Ricin A chain (RTA)
1j1m, 1ift, 2aai, 1rtc – RTA
3lc9, 3mk9, 2vc4, 1uq4, 1uq5, 1obs, 3bjg, 3srp – RTA (mutant)
Ricin A chain binary complexes
3px8 – RTA preproricin + 7-carboxy-pterin
1br5, 1br6 - RTA + pterin derivative
3px9 - RTA preproricin + furanylmethyl-carbamoyl-pterin
3lc9, 3mk9, 2vc4, 1uq4, 1uq5, 1obs – RTA (mutant)
3hio – RTA + tetranucleotide
3ej5, 1il5 – RTA pyrimidine derivative
2p8n, 1ifs – RTA + adenine
2pjo, 2r2x – RTA + urea derivative
2r3d – RTA + acetamide
2vc3 - RTA (mutant) + acetate
1il3, 1il4, 1il9 – RTA + guanine derivative
1ifu, 1fmp – RTA + formycin
1obt - RTA (mutant) + AMP
1apg – RTA + RNA
3px8 – RTA + formycin monophosphate
Ricin B chain (RTB)
3nbc, 3nbd – CnRTB + lactose – Clitocybe nebularis
3nbe – CnRTB + lactose derivative
3phz – RTB + glycoside – Polyporus squamosus
Ricin A+B chains
2aai - RTA + RTB
3rtj - RTA + RTB + dinucleotide
See Also
References
- ↑ 1.0 1.1 1.2 1.3 Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J. 1994 Feb;8(2):201-8. PMID:8119491
- ↑ 2.0 2.1 2.2 Montfort W, Villafranca JE, Monzingo AF, Ernst SR, Katzin B, Rutenber E, Xuong NH, Hamlin R, Robertus JD. The three-dimensional structure of ricin at 2.8 A. J Biol Chem. 1987 Apr 15;262(11):5398-403. PMID:3558397
- ↑ Weston SA, Tucker AD, Thatcher DR, Derbyshire DJ, Pauptit RA. X-ray structure of recombinant ricin A-chain at 1.8 A resolution. J Mol Biol. 1994 Dec 9;244(4):410-22. PMID:7990130 doi:http://dx.doi.org/10.1006/jmbi.1994.1739
- ↑ Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987 Apr 9-15;326(6113):624-6. PMID:3561502 doi:http://dx.doi.org/10.1038/326624a0
- ↑ 5.0 5.1 Rapak A, Falnes PO, Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):3783-8. PMID:9108055
- ↑ Holmberg L, Nygard O. Depurination of A4256 in 28 S rRNA by the ribosome-inactivating proteins from barley and ricin results in different ribosome conformations. J Mol Biol. 1996 May 31;259(1):81-94. PMID:8648651 doi:10.1006/jmbi.1996.0303
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Ann Taylor, Joel L. Sussman, Douglas Read, Wayne Decatur, David Canner, Angel Herraez, Jaime Prilusky, Alexander Berchansky, Andrea Gorrell