Sandbox Reserved 686
From Proteopedia
| Line 13: | Line 13: | ||
==Mechanism== | ==Mechanism== | ||
| - | The <scene name='Sandbox_Reserved_686/Full_basic_protein_only_ivm/2'>glutamate-gated chloride channel</scene> is made up of <scene name='Sandbox_Reserved_686/ | + | The <scene name='Sandbox_Reserved_686/Full_basic_protein_only_ivm/2'>glutamate-gated chloride channel</scene> is made up of <scene name='Sandbox_Reserved_686/Five_homologous_subunits/1'>five homologous subunits</scene>, as displayed by the different colorings. All the subunits are conserved structurally, but not necessarily in their amino acid sequence. Each monomer has <scene name='Sandbox_Reserved_686/Secondary_structures/2'>two domains</scene>. The extracellular domain is mainly β-pleated sheets, shown pink in the structure. The transmembrane portion of the ion channel, on each monomer, has four α-helices, colored blue. The α-helical portion of the subunit would not be accessible to solvent. The accessibility is not able to be shown in the 3D imagery. |
Ivermectin interacts within the lipid bilayer on the extracellular half. It inserts itself <scene name='Sandbox_Reserved_686/Between_subunits/1'>between two subunits</scene>, in the α-helices, to stabilize the open conformation of the ion channel (Ivermectin is brown, between a pink and tan subunit). There is a slight gapping evident between the two subunits, confirming that Ivermectin is opening up the channel. It is thought that the <scene name='Sandbox_Reserved_686/Serine_interaction/2'>interaction between serine 260 and Ivermectin</scene> is important in pulling those subunits open. With the subunits spread apart, the channel stays open longer, and has a higher affinity for glutamate. | Ivermectin interacts within the lipid bilayer on the extracellular half. It inserts itself <scene name='Sandbox_Reserved_686/Between_subunits/1'>between two subunits</scene>, in the α-helices, to stabilize the open conformation of the ion channel (Ivermectin is brown, between a pink and tan subunit). There is a slight gapping evident between the two subunits, confirming that Ivermectin is opening up the channel. It is thought that the <scene name='Sandbox_Reserved_686/Serine_interaction/2'>interaction between serine 260 and Ivermectin</scene> is important in pulling those subunits open. With the subunits spread apart, the channel stays open longer, and has a higher affinity for glutamate. | ||
With the channel stabilized in the open conformation by Ivermectin, chloride ions can freely flow into the cell. When the chloride ions are continually flowing, the polarization of the membrane is lost. This is how the parasitic microfilariae are killed. Their neurotransmission is severely inhibited. The microfilariae eventually die of flaccid paralysis. Ivermectin is often prescribed along with doxycycline in order to kill the ''Wolbachia'', as well. | With the channel stabilized in the open conformation by Ivermectin, chloride ions can freely flow into the cell. When the chloride ions are continually flowing, the polarization of the membrane is lost. This is how the parasitic microfilariae are killed. Their neurotransmission is severely inhibited. The microfilariae eventually die of flaccid paralysis. Ivermectin is often prescribed along with doxycycline in order to kill the ''Wolbachia'', as well. | ||
Revision as of 22:33, 3 May 2013
| This Sandbox is Reserved from 30/01/2013, through 30/12/2013 for use in the course "Biochemistry II" taught by Hannah Tims at the Messiah College. This reservation includes Sandbox Reserved 686 through Sandbox Reserved 700. |
To get started:
More help: Help:Editing |
Ivermectin
|
Background
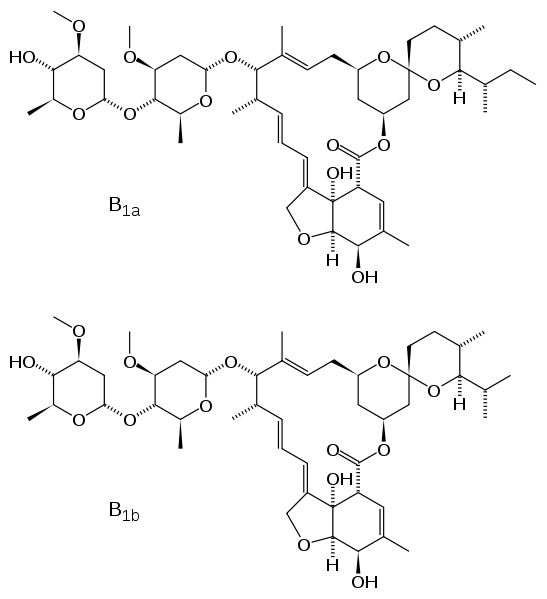
Ivermectin is an important pharmaceutical, used mainly as an anti-helminthic agent. It is a macrocyclic lactone, evidenced by its structure above, and a member of the larger category of avermectins. Ivermectin was discovered from Streptomyces avermitilis. One of the most common ways Ivermectin is used, is to treat Onchocerciasis. Onchocerciasis, better known as River Blindness, is the second leading cause of infectious blindness in the world.
O. volvulus and Wolbachia are the infectious agents involved in this disease. Wolbachia, the bacterial symbiont of O. volvulus, is released when the worm dies and causes inflammation and keratitis. Ivermectin work to kills the O. volvulus microfilariae and prevent the female macrofilariae from producing more microfilariae. The drug interacts with the glutamate-gated chloride channel to accomplish this.
Mechanism
The is made up of , as displayed by the different colorings. All the subunits are conserved structurally, but not necessarily in their amino acid sequence. Each monomer has . The extracellular domain is mainly β-pleated sheets, shown pink in the structure. The transmembrane portion of the ion channel, on each monomer, has four α-helices, colored blue. The α-helical portion of the subunit would not be accessible to solvent. The accessibility is not able to be shown in the 3D imagery.
Ivermectin interacts within the lipid bilayer on the extracellular half. It inserts itself , in the α-helices, to stabilize the open conformation of the ion channel (Ivermectin is brown, between a pink and tan subunit). There is a slight gapping evident between the two subunits, confirming that Ivermectin is opening up the channel. It is thought that the is important in pulling those subunits open. With the subunits spread apart, the channel stays open longer, and has a higher affinity for glutamate.
With the channel stabilized in the open conformation by Ivermectin, chloride ions can freely flow into the cell. When the chloride ions are continually flowing, the polarization of the membrane is lost. This is how the parasitic microfilariae are killed. Their neurotransmission is severely inhibited. The microfilariae eventually die of flaccid paralysis. Ivermectin is often prescribed along with doxycycline in order to kill the Wolbachia, as well.