Sandbox Reserved 696
From Proteopedia
| Line 19: | Line 19: | ||
== NMDAR Binding Sites == | == NMDAR Binding Sites == | ||
| - | Each NMDAR subunit binds a <scene name='Sandbox_Reserved_696/Ligand_and_protein/2'>ligand</scene> or an agonist in the center of the subunit. In the case of NMDARs the agonist is often N-methyl-D-asparate. The <scene name='Sandbox_Reserved_696/Ligand_and_protein_in_subunit/2'>amino acids</scene> of each subunit that interact with the glycine are Gly 141, Ser 142, Leu 90, and Ser 91. These amino acids interact with the glutamate on one side, and the ligand enters the subunit from the opposite side. The amino acid interactions are better visualized <scene name='Sandbox_Reserved_696/Ligand_aa/1'>here</scene>. | + | Each NMDAR subunit binds a <scene name='Sandbox_Reserved_696/Ligand_and_protein/2'>ligand</scene> or an agonist in the center of the subunit. In the case of NMDARs the agonist is often N-methyl-D-asparate. The <scene name='Sandbox_Reserved_696/Ligand_and_protein_in_subunit/2'>amino acids</scene> of each subunit that interact with the glycine are Gly 141, Ser 142, Leu 90, and Ser 91. These amino acids interact with the glutamate on one side, and the ligand enters the subunit from the opposite side. The amino acid interactions are better visualized <scene name='Sandbox_Reserved_696/Ligand_aa/1'>here</scene>. These amino acids are highly conserved. |
== NMDAR Ion Channel == | == NMDAR Ion Channel == | ||
| - | When a ligand binds, the subunit undergoes a conformational change. The conformational changes of the two subunits create a central <scene name='Sandbox_Reserved_696/Pore_in_ribbon_view/2'>channel</scene> that is not present when the ligand is unbound. This central channel is a non-selective cation channel. It allows Na+, Ca2+ and K+ to flow through the channel into the cell. | + | When a ligand binds, the subunit undergoes a conformational change. The conformational changes of the two subunits create a central <scene name='Sandbox_Reserved_696/Pore_in_ribbon_view/2'>channel</scene> that is not present when the ligand is unbound. This central channel is wider than most cation channels, so it is a non-selective cation channel. It allows Na+, Ca2+ and K+ to flow through the channel into the cell. The channel has four-fold symmetry and 16 alpha helices that make up the channel. There are <scene name='Sandbox_Reserved_696/Polar_channel/2'>polar residues</scene> in the channel that interact and encourage the cations through the pore. The influx of Na+ attributes to the buildup of positive charge that stimulates an action potential for a nerve impulse. The other cations, especially Ca2+, activate second messengers to stimulate cellular cascades that lead to transcription of certain genes. After the conformation change, an <scene name='Sandbox_Reserved_696/Space_filling/2'>antagonist</scene> can then bind to the entrance of the channel to prevent the <scene name='Sandbox_Reserved_696/Solvent/2'>influx</scene> of cations and subsequent cellular response. The <scene name='Sandbox_Reserved_696/Interactions_w_antagonist/2'>amino acids</scene> that interact with the antagonists are Ser 108, Leu 109, Ser 217, Phe 106, Pro 105, and Lys 218. Each subunit contains these <scene name='Sandbox_Reserved_696/Aa_with_anatag_highlighted/1'>amino acids</scene>. Each subunit binds the antagonist on both sides. |
| - | + | ||
| - | The <scene name='Sandbox_Reserved_696/Interactions_w_antagonist/2'>amino acids</scene> that interact with the antagonists are Ser 108, Leu 109, Ser 217, Phe 106, Pro 105, and Lys 218. Each subunit contains these amino acids. Each subunit | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 22:36, 3 May 2013
| This Sandbox is Reserved from 30/01/2013, through 30/12/2013 for use in the course "Biochemistry II" taught by Hannah Tims at the Messiah College. This reservation includes Sandbox Reserved 686 through Sandbox Reserved 700. |
To get started:
More help: Help:Editing |
Contents |
Memantine
Background
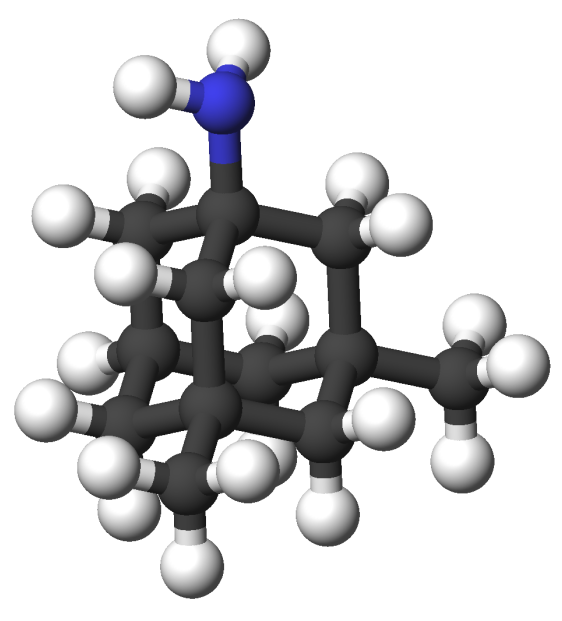
Memantine, pictured below, is an antagonist of N-methyl-D-asparate Receptors (NMDARs), a type of ionotropic glutamate receptor. Such receptors are found primarily on neurons. Memantine is the leading Alzheimer's drug used to prevent memory and learning capacity decline. Prescribed doses of memantine allows normal physiological activity of NMDARs but prevents overstimulation. Overstimulation of glutamate receptors causes a phenomenon called excitotoxicity, where the neuron ultimately dies. When groups of cells are subject to excitotoxicity, large portions of the brain die resulting in severe brain damage. This death of brain tissues are believed to cause learning inability and memory loss. By preventing overstimlation, Memantine aims to prevent excitotoxicity and the resulting diminish in learning inability and memory loss.
NMDAR Structure
NMDARs have two nearly symmetrical , NR1 and NR2. These subunits consist mainly of closely interacting alpha helices. Each subunit acts as a distinct functional unit, and each subunit binds a ligand separately. The subunits interact neatly with one another, with of each subunit interacting with polar portion of the opposite subunit and nonpolar portions interacting likewise.
|
NMDAR Binding Sites
Each NMDAR subunit binds a or an agonist in the center of the subunit. In the case of NMDARs the agonist is often N-methyl-D-asparate. The of each subunit that interact with the glycine are Gly 141, Ser 142, Leu 90, and Ser 91. These amino acids interact with the glutamate on one side, and the ligand enters the subunit from the opposite side. The amino acid interactions are better visualized . These amino acids are highly conserved.
NMDAR Ion Channel
When a ligand binds, the subunit undergoes a conformational change. The conformational changes of the two subunits create a central that is not present when the ligand is unbound. This central channel is wider than most cation channels, so it is a non-selective cation channel. It allows Na+, Ca2+ and K+ to flow through the channel into the cell. The channel has four-fold symmetry and 16 alpha helices that make up the channel. There are in the channel that interact and encourage the cations through the pore. The influx of Na+ attributes to the buildup of positive charge that stimulates an action potential for a nerve impulse. The other cations, especially Ca2+, activate second messengers to stimulate cellular cascades that lead to transcription of certain genes. After the conformation change, an can then bind to the entrance of the channel to prevent the of cations and subsequent cellular response. The that interact with the antagonists are Ser 108, Leu 109, Ser 217, Phe 106, Pro 105, and Lys 218. Each subunit contains these . Each subunit binds the antagonist on both sides.