We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Non-polymerizable monomeric actin
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
===Nucleotide-dependent structural changes in the nucleotide-binding cleft of AP-actin=== | ===Nucleotide-dependent structural changes in the nucleotide-binding cleft of AP-actin=== | ||
| - | <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_scene2/2'>Morph of AP-actin showing conformational changes between actin bound to ATP</scene> (PDB entry [[ | + | <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_scene2/2'>Morph of AP-actin showing conformational changes between actin bound to ATP</scene> (PDB entry [[2hf4]]) and ADP (PDB entry [[2hf3]]). Nucleotide is not shown. |
[[Image:2HF4 REDO OF MORPH STILL IMAGE.png|300px|left|thumb| Sensor loop of AP-actin bound to ADP (grey) and ATP (white)]] | [[Image:2HF4 REDO OF MORPH STILL IMAGE.png|300px|left|thumb| Sensor loop of AP-actin bound to ADP (grey) and ATP (white)]] | ||
| Line 21: | Line 21: | ||
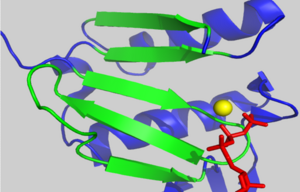
[[Image:2hf4 d loop crystal packing.png|250px|left|thumb| Crystal packing interactions between ADP-bound AP-actin, (grey ribbon) and neighboring residues in subdomain 2 of an adjacent monomer in the crystal within 15 angstroms of the D-loop in the unit cell (blue ribbons). Residues 39-50 are shown in red.]] | [[Image:2hf4 d loop crystal packing.png|250px|left|thumb| Crystal packing interactions between ADP-bound AP-actin, (grey ribbon) and neighboring residues in subdomain 2 of an adjacent monomer in the crystal within 15 angstroms of the D-loop in the unit cell (blue ribbons). Residues 39-50 are shown in red.]] | ||
{{clear}} | {{clear}} | ||
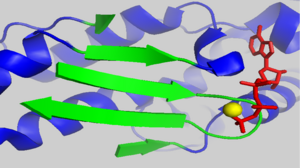
| - | [[Image:1J6Z d loop crystal packing.png|250px|left|thumb| Crystal packing interactions between ADP-actin, complexed with TMR (grey ribbon)(PDB entry [[ | + | [[Image:1J6Z d loop crystal packing.png|250px|left|thumb| Crystal packing interactions between ADP-actin, complexed with TMR (grey ribbon)(PDB entry [[1j6z]]) and neighboring residues in subdomain 2 of an adjacent monomer in the crystal within 15 angstroms of the D-loop in the unit cell (blue ribbons). Residues 39-50 are shown in red.]] |
{{clear}} | {{clear}} | ||
===The D-loop=== | ===The D-loop=== | ||
<scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_d_loop_morph/2'>Morph of subdomain 2 of actin complexed with TMR in the ADP state and actin complexed with DNAse I in the ATP state</scene> <ref name="TMR"/>. | <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_d_loop_morph/2'>Morph of subdomain 2 of actin complexed with TMR in the ADP state and actin complexed with DNAse I in the ATP state</scene> <ref name="TMR"/>. | ||
| - | There is some controversy over whether or not the D-loop undergoes structural changes upon actin binding ATP. In the structure of AP-actin, the D-loop is disordered in both the ATP and ADP-bound state. Also, there is no evidence that structural changes in the nucleotide binding cleft propagate to subdomain 2. This argues that the D-loop remains disordered in both states. However, other groups show large ATP-dependent structural changes in the D-loop<ref name="TMR">PMID:11474115</ref>. This is illustrated, right, in a subdomain 2 morph of actin complexed with tetramethylrhodamine (TMR) in the ADP-bound state, (PDB entry [[ | + | There is some controversy over whether or not the D-loop undergoes structural changes upon actin binding ATP. In the structure of AP-actin, the D-loop is disordered in both the ATP and ADP-bound state. Also, there is no evidence that structural changes in the nucleotide binding cleft propagate to subdomain 2. This argues that the D-loop remains disordered in both states. However, other groups show large ATP-dependent structural changes in the D-loop<ref name="TMR">PMID:11474115</ref>. This is illustrated, right, in a subdomain 2 morph of actin complexed with tetramethylrhodamine (TMR) in the ADP-bound state, (PDB entry [[1j6z]]) and actin complexed with DNAase I in the ATP-bound state, (PDB entry [[1atn]])<ref name="TMR"/>. These structures revile that the D-loop is disordered when actin is bound to ATP, and transitions to an alpha-helix in the ADP-bound state. It has been suggested that the alpha helix in the ADP-bound state results from crystal packing. In support of this, actin complexed with TMR in the ADP state shows extensive neighboring contacts around the D-loop (shown left, lower panel). In contrast, AP-actin, (PDB entry [[2hf3]]), shows far fewer crystal contacts around the D-loop (shown left, upper panel). These crystal contacts have been proposed to result in the nucleotide-dependent structural changes in the D-loop observed in some structures. In further support of this, the sequence of the D-loop, HQGVMVGMG, has a low propensity to form an alpha helix. However, molecular dynamic simulations show that the D-loop favors the alpha helix conformation in the ADP state, and not the ATP or ADP-Pi states <ref name="zheng">PMID:17526584</ref>. This study supports a model where small perturbations in the active site shift the equilibrium of the D-loop between the flexible coil and helix state. Further studies are needed to resolve this question. |
== The actin fold == | == The actin fold == | ||
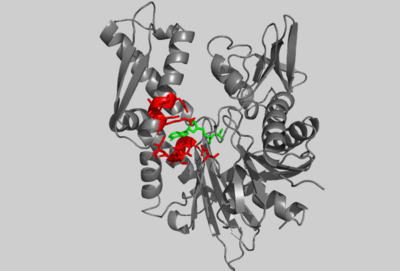
| - | [[Image:2HF4 COMP 1HSC IMAGE.png|400px|left|thumb| Crystal structure of ATP-bound AP-actin, (PDB entry | + | [[Image:2HF4 COMP 1HSC IMAGE.png|400px|left|thumb| Crystal structure of ATP-bound AP-actin, (PDB entry [[2hf4]]), with selected residues shown in red. ATP is shown in green.]] |
{{clear}} | {{clear}} | ||
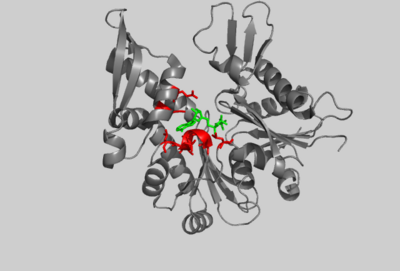
| - | [[Image:1HSC COMP 2HF4 IMAGE.png|400px|left|thumb| Crystal structure of the amino-terminal 44K ATPase fragment of the 70K bovine Hsc70 protein, (PDB entry [[ | + | [[Image:1HSC COMP 2HF4 IMAGE.png|400px|left|thumb| Crystal structure of the amino-terminal 44K ATPase fragment of the 70K bovine Hsc70 protein, (PDB entry [[3hsc]]), bound to ADP. Selected residues are shown in red. ADP is shown in green.]] |
{{clear}} | {{clear}} | ||
| - | [[Image:1JCG COMP 2HF4 IMAGE.png|400px|left|thumb| Crystal structure of MreB from Thermotoga Maritima bound to AMP.PNP, (PDB entry [[ | + | [[Image:1JCG COMP 2HF4 IMAGE.png|400px|left|thumb| Crystal structure of MreB from Thermotoga Maritima bound to AMP.PNP, (PDB entry [[1jcg]]). Selected residues are shown in red. AMP.PNP is shown in green.]] |
{{clear}} | {{clear}} | ||
| - | Through structural comparisons, it’s possible to identify evolutionary relationships among proteins with common folds. The core structure of actin is strikingly similar to the structure of Hsc70 and MreB which both bind ATP with a common motif. Hsc70 is a member of the 70-kDa heat-shock family Hsp70 which binds to unfolded proteins and hydrolyzes ATP. Exchange factors recycle Hsc70 by stimulating ATPase activity thereby mediating dissociation from the protein substrate<ref name="Buchberger">PMID:7656024</ref>. On the right, the crystal structure of a 44-kDa amino-terminal fragment of Hsc70 (PDB entry [[ | + | Through structural comparisons, it’s possible to identify evolutionary relationships among proteins with common folds. The core structure of actin is strikingly similar to the structure of Hsc70 and MreB which both bind ATP with a common motif. Hsc70 is a member of the 70-kDa heat-shock family Hsp70 which binds to unfolded proteins and hydrolyzes ATP. Exchange factors recycle Hsc70 by stimulating ATPase activity thereby mediating dissociation from the protein substrate<ref name="Buchberger">PMID:7656024</ref>. On the right, the crystal structure of a 44-kDa amino-terminal fragment of Hsc70 (PDB entry [[3hsc]]) is shown that retains ATPase activity but is incapable of binding proteins<ref name="Flaherty">PMID: 2143562</ref>. AP-actin, in blue, is shown aligned to this structure with functionally and structurally conserved residues in red. |
Subdomains 1 and 3 of actin are composed of a five-stranded β-sheet surrounded by α-helicies. These domains are highly conserved in Hsc70. The interface between subdomains 1, 3, and 4 are required in both structures to bind nucleotide. The adenosine group of ATP fits into a hydrophobic pocket between subdomains 3 and 4, formed by two helices (AP-actin: residues 210-214, 302-306, 336 and Hsc70: residues 268-272, 339-343, 366 as shown in red for both structures). The ribose is bound by Glu214 in actin and Glu268 in Hsc70. These residues form salt bridges with adjacent arginines in both structures. | Subdomains 1 and 3 of actin are composed of a five-stranded β-sheet surrounded by α-helicies. These domains are highly conserved in Hsc70. The interface between subdomains 1, 3, and 4 are required in both structures to bind nucleotide. The adenosine group of ATP fits into a hydrophobic pocket between subdomains 3 and 4, formed by two helices (AP-actin: residues 210-214, 302-306, 336 and Hsc70: residues 268-272, 339-343, 366 as shown in red for both structures). The ribose is bound by Glu214 in actin and Glu268 in Hsc70. These residues form salt bridges with adjacent arginines in both structures. | ||
A comparison of these two structures shows that not only is the nucleotide binding cleft conserved but also helix packing against β-sheets in subdomains 1 and 3. This same mode of structural conservation is described for hexokinase and glycerol kinase<ref name="Kabsch">PMID: 7781919</ref>. | A comparison of these two structures shows that not only is the nucleotide binding cleft conserved but also helix packing against β-sheets in subdomains 1 and 3. This same mode of structural conservation is described for hexokinase and glycerol kinase<ref name="Kabsch">PMID: 7781919</ref>. | ||
| - | While Hsp70 and sugar kinases show ~50% sequence identity, the bacterial cytoskeletal protein MreB shares less than 15% sequence identity. However, a comparison of the crystal structures of actin and MreB reveals high structural homology. Like actin, MreB contains two domains composed of a five-stranded β-sheet. These domains are connected by a helix, similar to actin. Subdomains 2 and 4 in actin are more diverse in the actin superfamily and share little structural homology with Hsp70 and sugar kinases. Remarkably, MreB shares the same topology as actin in these domains<ref name="Ent">PMID:11544518</ref>. MreB can be superimposed on actin (PDB entry [[ | + | While Hsp70 and sugar kinases show ~50% sequence identity, the bacterial cytoskeletal protein MreB shares less than 15% sequence identity. However, a comparison of the crystal structures of actin and MreB reveals high structural homology. Like actin, MreB contains two domains composed of a five-stranded β-sheet. These domains are connected by a helix, similar to actin. Subdomains 2 and 4 in actin are more diverse in the actin superfamily and share little structural homology with Hsp70 and sugar kinases. Remarkably, MreB shares the same topology as actin in these domains<ref name="Ent">PMID:11544518</ref>. MreB can be superimposed on actin (PDB entry [[1atn]]) with an r.m.s. deviation of 3.7 Å over 310 C atoms<ref name="Holm">PMID: 8377180</ref>. Also, MreB can be superimposed on Hsp70 (PDB entry [[1hpm]]) with an r.m.s. deviation of 3.4 Å over 299 C atoms. However, Hsp70 has a large insert in subdomain 4 not present in actin or MreB. This result suggests that MreB is closely related to Hsp70. Shown right is a crystal structure of MreB (PDB entry [[1jcg]]) bound to AMP.PNP and aligned with AP-actin. Structurally conserved residues corresponding to those in actin are indicated in red. They include residues at the interface between subdomains 1 and 3, and a functionally conserved glutamic acid at position 204. This residue in MreB forms a salt bridge with an adjacent lysine residue similar to glutamic acid 214 in actin and glutamic acid 268 in Hsc70. |
The presence of a structurally conserved ATP-binding motif in the actin superfamily and MreB suggests that these proteins are likely the result of divergent evolution from a common ancestor. | The presence of a structurally conserved ATP-binding motif in the actin superfamily and MreB suggests that these proteins are likely the result of divergent evolution from a common ancestor. | ||
Revision as of 08:04, 8 May 2013
| |||||||||||
See Also
References
- ↑ Rould MA, Wan Q, Joel PB, Lowey S, Trybus KM. Crystal structures of expressed non-polymerizable monomeric actin in the ADP and ATP states. J Biol Chem. 2006 Oct 20;281(42):31909-19. Epub 2006 Aug 18. PMID:16920713 doi:M601973200
- ↑ 2.0 2.1 2.2 2.3 Otterbein LR, Graceffa P, Dominguez R. The crystal structure of uncomplexed actin in the ADP state. Science. 2001 Jul 27;293(5530):708-11. PMID:11474115 doi:10.1126/science.1059700
- ↑ Zheng X, Diraviyam K, Sept D. Nucleotide effects on the structure and dynamics of actin. Biophys J. 2007 Aug 15;93(4):1277-83. Epub 2007 May 25. PMID:17526584 doi:10.1529/biophysj.107.109215
- ↑ Buchberger A, Schroder H, Buttner M, Valencia A, Bukau B. A conserved loop in the ATPase domain of the DnaK chaperone is essential for stable binding of GrpE. Nat Struct Biol. 1994 Feb;1(2):95-101. PMID:7656024
- ↑ Flaherty KM, DeLuca-Flaherty C, McKay DB. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623-8. PMID:2143562 doi:http://dx.doi.org/10.1038/346623a0
- ↑ Kabsch W, Holmes KC. The actin fold. FASEB J. 1995 Feb;9(2):167-74. PMID:7781919
- ↑ van den Ent F, Amos LA, Lowe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001 Sep 6;413(6851):39-44. PMID:11544518 doi:http://dx.doi.org/10.1038/35092500
- ↑ Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J Mol Biol. 1993 Sep 5;233(1):123-38. PMID:8377180 doi:http://dx.doi.org/10.1006/jmbi.1993.1489
- ↑ Aurora R, Rose GD. Helix capping. Protein Sci. 1998 Jan;7(1):21-38. PMID:9514257 doi:10.1002/pro.5560070103