This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
2h24
From Proteopedia
(New page: 200px<br /> <applet load="2h24" size="450" color="white" frame="true" align="right" spinBox="true" caption="2h24, resolution 2.0Å" /> '''Crystal structure of...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:2h24.gif|left|200px]]<br /> | + | [[Image:2h24.gif|left|200px]]<br /><applet load="2h24" size="350" color="white" frame="true" align="right" spinBox="true" |
| - | <applet load="2h24" size=" | + | |
caption="2h24, resolution 2.0Å" /> | caption="2h24, resolution 2.0Å" /> | ||
'''Crystal structure of human IL-10'''<br /> | '''Crystal structure of human IL-10'''<br /> | ||
==Overview== | ==Overview== | ||
| - | Interleukin-10 receptor 2 (IL-10R2) is a critical component of the | + | Interleukin-10 receptor 2 (IL-10R2) is a critical component of the IL-10.IL-10R1.IL-10R2 complex which regulates IL-10-mediated immunomodulatory responses. The ternary IL-10 signaling complex is assembled in a sequential order with the IL-10.IL-10R1 interaction occurring first followed by engagement of the IL-10R2 chain. In this study we map the IL-10R2 binding site on IL-10 using surface plasmon resonance and cell-based assays. Critical IL-10R2 binding residues are located in helix A adjacent to the previously identified IL-10R1 recognition surface. Interestingly, IL-10R2 binding residues located in the N-terminal end of helix A exhibit large structural differences between unbound cIL-10 and cIL-10.IL-10R1 crystal structures. This suggests IL-10R1-induced conformational changes regulate IL-10R2 binding and assembly of the ternary IL-10.IL-10R1.IL-10R2 complex. The basic mechanistic features of the assembly process are likely shared by six additional class-2 cytokines (viral IL-10s, IL-22, IL-26, IL-28A, IL28B, and IL-29) to promote IL-10R2 binding to six additional receptor complexes. These studies highlight the importance of structure in regulating low affinity protein-protein interactions and IL-10 signal transduction. |
==Disease== | ==Disease== | ||
| Line 11: | Line 10: | ||
==About this Structure== | ==About this Structure== | ||
| - | 2H24 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http:// | + | 2H24 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2H24 OCA]. |
==Reference== | ==Reference== | ||
| Line 17: | Line 16: | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| - | [[Category: Walter, M | + | [[Category: Walter, M R.]] |
| - | [[Category: Yoon, S | + | [[Category: Yoon, S I.]] |
[[Category: alpha-helix bundle]] | [[Category: alpha-helix bundle]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 17:37:24 2008'' |
Revision as of 15:37, 21 February 2008
|
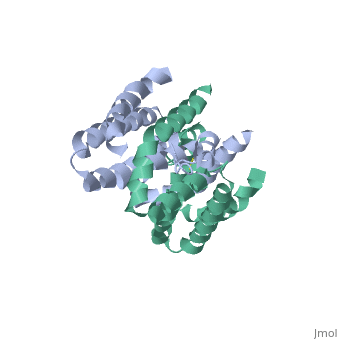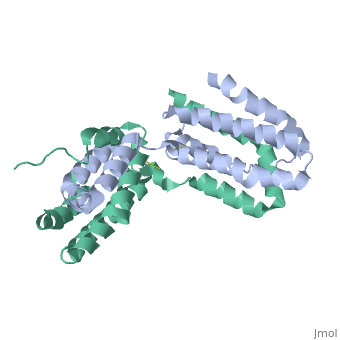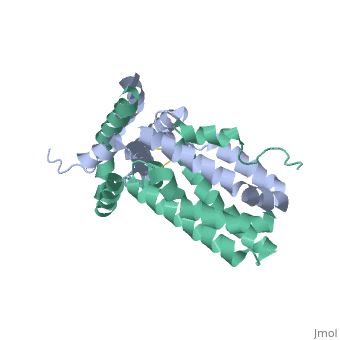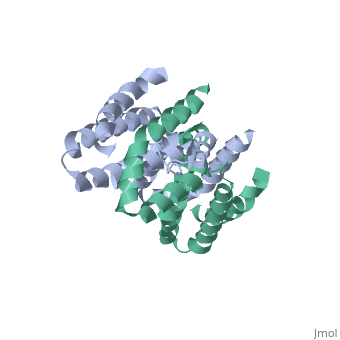
Crystal structure of human IL-10
Contents |
Overview
Interleukin-10 receptor 2 (IL-10R2) is a critical component of the IL-10.IL-10R1.IL-10R2 complex which regulates IL-10-mediated immunomodulatory responses. The ternary IL-10 signaling complex is assembled in a sequential order with the IL-10.IL-10R1 interaction occurring first followed by engagement of the IL-10R2 chain. In this study we map the IL-10R2 binding site on IL-10 using surface plasmon resonance and cell-based assays. Critical IL-10R2 binding residues are located in helix A adjacent to the previously identified IL-10R1 recognition surface. Interestingly, IL-10R2 binding residues located in the N-terminal end of helix A exhibit large structural differences between unbound cIL-10 and cIL-10.IL-10R1 crystal structures. This suggests IL-10R1-induced conformational changes regulate IL-10R2 binding and assembly of the ternary IL-10.IL-10R1.IL-10R2 complex. The basic mechanistic features of the assembly process are likely shared by six additional class-2 cytokines (viral IL-10s, IL-22, IL-26, IL-28A, IL28B, and IL-29) to promote IL-10R2 binding to six additional receptor complexes. These studies highlight the importance of structure in regulating low affinity protein-protein interactions and IL-10 signal transduction.
Disease
Known diseases associated with this structure: Graft-versus-host disease, protection against OMIM:[124092], HIV-1, susceptibility to OMIM:[124092], Rheumatoid arthritis, progression of OMIM:[124092]
About this Structure
2H24 is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
Conformational changes mediate interleukin-10 receptor 2 (IL-10R2) binding to IL-10 and assembly of the signaling complex., Yoon SI, Logsdon NJ, Sheikh F, Donnelly RP, Walter MR, J Biol Chem. 2006 Nov 17;281(46):35088-96. Epub 2006 Sep 18. PMID:16982608
Page seeded by OCA on Thu Feb 21 17:37:24 2008