Serine protease
From Proteopedia
m (Serine Protease moved to Serine protease) |
|||
| Line 17: | Line 17: | ||
<applet load="2ptc" size="350" color="white" frame="true" align="right" spinBox="true" | <applet load="2ptc" size="350" color="white" frame="true" align="right" spinBox="true" | ||
| - | caption="β-Trypsin BPT1 complex with Ca+2 ion [[2ptc]]" /> | + | caption="β-Trypsin (magenta) BPT1 (wheat) complex with Ca+2 ion [[2ptc]]" /> |
==Trypsin-BPTI complex== | ==Trypsin-BPTI complex== | ||
Revision as of 08:13, 8 July 2013
|
Serine proteases, or proteinases, so called due to the presence of a serine residue in the active site, are a class of enzymes that catalyse the hydrolysis of peptide bonds in proteins.
Contents |
Thrombin
Thrombin is a "trypsin-like" serine protease. Its structure (PDB code 1ppb) is shown here with a peptide chloroketone inhibitor (PPACK). The thrombin A chain (cleaved N terminal fragement) is shown in cyan and the B chain is shown in red. The is made up of a catalytic triad of Ser195, His57 and Asp102, backed up by Ser214. The peptide chloroketone inhibitor (PPACK) is shown in purple. A closeup shows the at which the sidechain of Asp194 makes a salt link with the N-terminus at residue 16, newly formed when the A chain is cleaved in the zymogen-to-enzyme activation process. The specificity pocket is on one side of the throat of the domain 2 beta barrel, and the activation site is close next to it.
The B chain consists of . As is true for all of the "trypsin-like" serine proteases, each of the two thrombin domains consists mainly of a 6-stranded, antiparallel beta barrel. The specificity pocket (here filled with the Lys sidechain of the PPACK inhibitor) is in one side of the throat of the domain 2beta barrel, and the activation site is close next to it.
3D structures of thrombin
THis is protein fro CHEM361.
|
Trypsin-BPTI complex
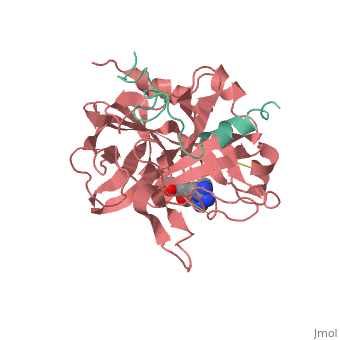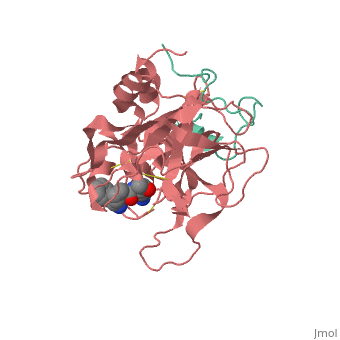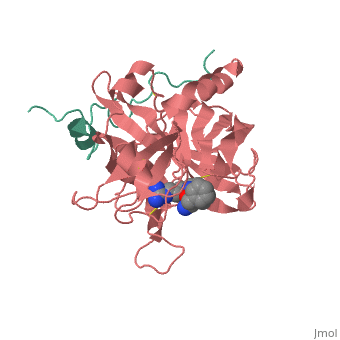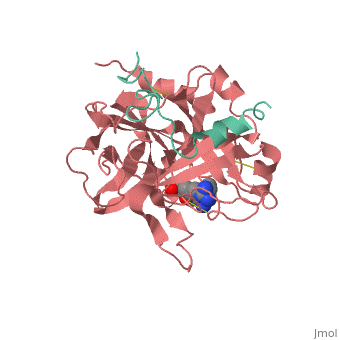
The trypsin backbone is shown in pink and the trypsin inhibitor, BPTI, in yellow (PDB code 2ptc). The residues [Ser195-His57-Asp102-Ser214] are shown in green, the disulfide bond between residues 14-38 is shown in yellow and the Lys 15 sidechain at the specificity site in pink.
Gilman suc-AAPK-trypsin
Content Donators
- Content for this page has been included and adapted with permission from Jane S. and David C. Richardson's http://kinemage.biochem.duke.edu/
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Ailie McGregor, Alexander Berchansky, Ann Taylor, Harry Greenblatt, Eran Hodis, Jaime Prilusky