Alcohol dehydrogenase
From Proteopedia
| Line 1: | Line 1: | ||
| - | < | + | <StructureSection load='1htb' size='450' side='right' scene='' caption=''> |
| + | |||
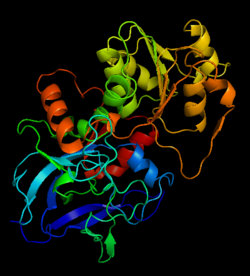
[[Image:1htb2.png|thumb|left|250px|Structure of Alcohol Dehydrogenase]]'''Alcohol dehydrogenase''' (ADH, EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=1.1.1.1 1.1.1.1]) is an 80kDa enzyme that catalyzes the 4th step in the metabolism of fructose before [[glycolysis]]. In the 4th step, glyceraldehyde is converted to the glycolytic intermediate DHAP by the NADH-dependent, ADH catalyzed reduction to glycerol.<ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> ADH catalyzes the oxidation of primary and secondary alcohols to their corresponding aldehydes and ketones through a mechanism that involves the removal of a hydrogen. For detailed discussion of horse liver alcohol dehydrogenase see [[Horse Liver Alcohol Dehydrogenase]]. More detailed discussions in<br /> | [[Image:1htb2.png|thumb|left|250px|Structure of Alcohol Dehydrogenase]]'''Alcohol dehydrogenase''' (ADH, EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=1.1.1.1 1.1.1.1]) is an 80kDa enzyme that catalyzes the 4th step in the metabolism of fructose before [[glycolysis]]. In the 4th step, glyceraldehyde is converted to the glycolytic intermediate DHAP by the NADH-dependent, ADH catalyzed reduction to glycerol.<ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> ADH catalyzes the oxidation of primary and secondary alcohols to their corresponding aldehydes and ketones through a mechanism that involves the removal of a hydrogen. For detailed discussion of horse liver alcohol dehydrogenase see [[Horse Liver Alcohol Dehydrogenase]]. More detailed discussions in<br /> | ||
| Line 22: | Line 23: | ||
==Structure== | ==Structure== | ||
| - | + | ||
The initial scene (<scene name='Birrer_Sandbox_2/Whole_adh_molecule/3'>Domains of ADH</scene>) shows an overview of the molecule, allowing for a general look at the tertiary structure of alcohol dehydrogenase (it is complexed with Cl, Pyz, NAD, and Zn). A second scene (<scene name='Birrer_Sandbox_2/Close_look_at_ligand/2'>Closer Look at Subunit</scene>) shows a close view of the ligand within each subunit. Labels have been placed on NAD, CL, and Zn to clearly establish the structure. | The initial scene (<scene name='Birrer_Sandbox_2/Whole_adh_molecule/3'>Domains of ADH</scene>) shows an overview of the molecule, allowing for a general look at the tertiary structure of alcohol dehydrogenase (it is complexed with Cl, Pyz, NAD, and Zn). A second scene (<scene name='Birrer_Sandbox_2/Close_look_at_ligand/2'>Closer Look at Subunit</scene>) shows a close view of the ligand within each subunit. Labels have been placed on NAD, CL, and Zn to clearly establish the structure. | ||
| Line 46: | Line 47: | ||
<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> | <ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> | ||
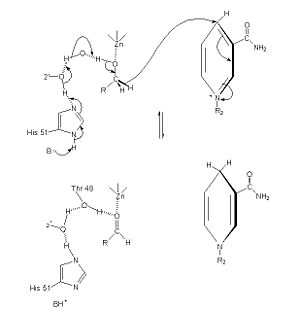
The of alcohol dehydrogenase reaction is as follows: CH3CH2OH + NAD+ -> CH3COH (acetaldehyde) + NADH + H+ (Note: The reaction is actually reversible although the arrow does not show it) <ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> The step-wise reduction mechanism for ADH is shown on the left. In the mechanism, His 51 is deprotonated and activated by a base catalyst. This allows histidine to accept a proton from NAD, which also draws a proton Thr 48. As a result of the proton transfer, the Thr is prepared to accept a proton from the alcohol substrate. While Thr accepts the proton, there is also a hydride transfer to NAD. The whole process can be summarized as the oxidation of an alcohol to an aldehyde in concert with the transfer of a hydride to NAD.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> | The of alcohol dehydrogenase reaction is as follows: CH3CH2OH + NAD+ -> CH3COH (acetaldehyde) + NADH + H+ (Note: The reaction is actually reversible although the arrow does not show it) <ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> The step-wise reduction mechanism for ADH is shown on the left. In the mechanism, His 51 is deprotonated and activated by a base catalyst. This allows histidine to accept a proton from NAD, which also draws a proton Thr 48. As a result of the proton transfer, the Thr is prepared to accept a proton from the alcohol substrate. While Thr accepts the proton, there is also a hydride transfer to NAD. The whole process can be summarized as the oxidation of an alcohol to an aldehyde in concert with the transfer of a hydride to NAD.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> | ||
| - | |||
The Mechanism for alcohol dehydrogenase follows an random bisubstrate mechanism.<ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> In the mechanism, the NAD+ and alcohol bind to the enzyme, so that the enzyme is now attached to the two subtrates. While attached, the hydrogen is formally transferred from the alcohol to NAD, resulting in the products NADH and a ketone or aldehyde. The two products are then released, and the enzyme has catalyzed the reaction. | The Mechanism for alcohol dehydrogenase follows an random bisubstrate mechanism.<ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> In the mechanism, the NAD+ and alcohol bind to the enzyme, so that the enzyme is now attached to the two subtrates. While attached, the hydrogen is formally transferred from the alcohol to NAD, resulting in the products NADH and a ketone or aldehyde. The two products are then released, and the enzyme has catalyzed the reaction. | ||
| - | |||
==Kinetics== | ==Kinetics== | ||
| - | |||
The alcohol dehydrogenase catalyzed aldehyde-NADH reaction show kinetics consistent with a random-order mechanism, and the rate-limiting step is the dissociation of the product enzyme-NAD+ complex. <ref>PMID: 4352908</ref> Alcohol dehydrogenase is more effective for smaller alcohol substrates, and it becomes less effective as substrate size increases. It is also more effective for primary than secondary alcohols.<ref>PMID: 4352908</ref> In a study where ADH was immobilized in tresyl-chloride-activate agarose, it was shown that the Michaelis-Menten model could not take into consideration all the constraints induced by the immobilization on the enzyme properties but that the Theorell-Chance model was more appropriate.<ref>PMID: 3769934</ref> | The alcohol dehydrogenase catalyzed aldehyde-NADH reaction show kinetics consistent with a random-order mechanism, and the rate-limiting step is the dissociation of the product enzyme-NAD+ complex. <ref>PMID: 4352908</ref> Alcohol dehydrogenase is more effective for smaller alcohol substrates, and it becomes less effective as substrate size increases. It is also more effective for primary than secondary alcohols.<ref>PMID: 4352908</ref> In a study where ADH was immobilized in tresyl-chloride-activate agarose, it was shown that the Michaelis-Menten model could not take into consideration all the constraints induced by the immobilization on the enzyme properties but that the Theorell-Chance model was more appropriate.<ref>PMID: 3769934</ref> | ||
| - | |||
| - | |||
==Regulation== | ==Regulation== | ||
| Line 62: | Line 58: | ||
==Tetrameric alcohol dehydrogenases== | ==Tetrameric alcohol dehydrogenases== | ||
| - | <StructureSection load='3fsr' size='500' frame='true' align='right' scene='3fsr/Cv/2' > | ||
The NADP<sup>+</sup>-dependent alcohol dehydrogenases from the [http://en.wikipedia.org/wiki/Thermophile thermophile] ''Thermoanaerobacter brockii'' (TbADH), the [http://en.wikipedia.org/wiki/Mesophile mesophilic] [http://en.wikipedia.org/wiki/Bacteria bacterium] [http://en.wikipedia.org/wiki/Clostridium_beijerinckii ''Clostridium beijerinckii''] (CbADH), and the [http://en.wikipedia.org/wiki/Protozoa protozoan] [http://en.wikipedia.org/wiki/Parasitism parasite] [http://en.wikipedia.org/wiki/Entamoeba_histolytica ''Entamoeba histolytica''] (EhADH1) are <scene name='3fsr/Cv/3'>homotetrameric</scene> [http://en.wikipedia.org/wiki/Tetrameric_protein] ([http://en.wikipedia.org/wiki/Protein_subunit monomers] are colored in different colors) secondary alcohol dehydrogenases. Each <scene name='3fsr/Cv/4'>monomer</scene> of these alcohol dehydrogenases consists of two domains: the <scene name='3fsr/Cv/5'>cofactor-binding domain</scene> <font color='blueviolet'><b> (residues 154−294 for TbADH)</b></font> and the <scene name='3fsr/Cv/6'>catalytic domain</scene> (<font color='red'><b>residues 1−153 and 295−351 for TbADH</b></font>; contains [http://en.wikipedia.org/wiki/Zinc Zn<sup>2+</sup>] at the [http://en.wikipedia.org/wiki/Active_site active site]) separated by a deep cleft. Although, all these three ADHs revealed a high degree of [http://en.wikipedia.org/wiki/Conserved_sequence sequence conservation] (62-75% identity), them significantly differ in [http://en.wikipedia.org/wiki/Thermostability thermostability]. The [http://en.wikipedia.org/wiki/Cofactor_(biochemistry) cofactor]-binding domains (residues 153−295) of TbADH, CbADH, and EhADH1 were mutually <scene name='3fsr/Cv/7'>exchanged</scene> and 3 corresponding chimeras were constructed. | The NADP<sup>+</sup>-dependent alcohol dehydrogenases from the [http://en.wikipedia.org/wiki/Thermophile thermophile] ''Thermoanaerobacter brockii'' (TbADH), the [http://en.wikipedia.org/wiki/Mesophile mesophilic] [http://en.wikipedia.org/wiki/Bacteria bacterium] [http://en.wikipedia.org/wiki/Clostridium_beijerinckii ''Clostridium beijerinckii''] (CbADH), and the [http://en.wikipedia.org/wiki/Protozoa protozoan] [http://en.wikipedia.org/wiki/Parasitism parasite] [http://en.wikipedia.org/wiki/Entamoeba_histolytica ''Entamoeba histolytica''] (EhADH1) are <scene name='3fsr/Cv/3'>homotetrameric</scene> [http://en.wikipedia.org/wiki/Tetrameric_protein] ([http://en.wikipedia.org/wiki/Protein_subunit monomers] are colored in different colors) secondary alcohol dehydrogenases. Each <scene name='3fsr/Cv/4'>monomer</scene> of these alcohol dehydrogenases consists of two domains: the <scene name='3fsr/Cv/5'>cofactor-binding domain</scene> <font color='blueviolet'><b> (residues 154−294 for TbADH)</b></font> and the <scene name='3fsr/Cv/6'>catalytic domain</scene> (<font color='red'><b>residues 1−153 and 295−351 for TbADH</b></font>; contains [http://en.wikipedia.org/wiki/Zinc Zn<sup>2+</sup>] at the [http://en.wikipedia.org/wiki/Active_site active site]) separated by a deep cleft. Although, all these three ADHs revealed a high degree of [http://en.wikipedia.org/wiki/Conserved_sequence sequence conservation] (62-75% identity), them significantly differ in [http://en.wikipedia.org/wiki/Thermostability thermostability]. The [http://en.wikipedia.org/wiki/Cofactor_(biochemistry) cofactor]-binding domains (residues 153−295) of TbADH, CbADH, and EhADH1 were mutually <scene name='3fsr/Cv/7'>exchanged</scene> and 3 corresponding chimeras were constructed. | ||
| Line 73: | Line 68: | ||
{{Clear}} | {{Clear}} | ||
| - | |||
The <scene name='3fsr/Al1/2'>comparison</scene> of overall Cα backbone of all these chimeras (rmsd 0.45-0.65 Å) with those of the parent enzymes, did not reveal significant structural changes. So, the differences in the thermal stability of the chimeras and the parent enzymes could be caused by relatively small specific changes located at the important points of the NADP<sup>+</sup>-dependent alcohol dehydrogenases. For example see Cα superposition for the <font color='red'><b>X23<sub>(TET)</sub> chimera (red)</b></font> ([[3fpc]]) and its parent ADHs (<font color='blue'><b>TbADH, colored blue</b></font> ([[1ped]]), and <font color='lime'><b>EhADH1, colored lime</b></font> ([[1y9a]]). The [http://en.wikipedia.org/wiki/Root_mean_square_deviation RMSDs] of the TbADH−EhADH1, TbADH−Χ23<sub>(TET)</sub>, and EhADH1−Χ23<sub>(TET)</sub> were 0.68, 0.56, and 0.48 Å, respectively. | The <scene name='3fsr/Al1/2'>comparison</scene> of overall Cα backbone of all these chimeras (rmsd 0.45-0.65 Å) with those of the parent enzymes, did not reveal significant structural changes. So, the differences in the thermal stability of the chimeras and the parent enzymes could be caused by relatively small specific changes located at the important points of the NADP<sup>+</sup>-dependent alcohol dehydrogenases. For example see Cα superposition for the <font color='red'><b>X23<sub>(TET)</sub> chimera (red)</b></font> ([[3fpc]]) and its parent ADHs (<font color='blue'><b>TbADH, colored blue</b></font> ([[1ped]]), and <font color='lime'><b>EhADH1, colored lime</b></font> ([[1y9a]]). The [http://en.wikipedia.org/wiki/Root_mean_square_deviation RMSDs] of the TbADH−EhADH1, TbADH−Χ23<sub>(TET)</sub>, and EhADH1−Χ23<sub>(TET)</sub> were 0.68, 0.56, and 0.48 Å, respectively. | ||
| Line 85: | Line 79: | ||
{{Clear}} | {{Clear}} | ||
</StructureSection> | </StructureSection> | ||
| - | |||
==Additional Resources== | ==Additional Resources== | ||
Revision as of 09:33, 16 July 2013
| |||||||||||
Contents |
Additional Resources
For additional information, see: Carbohydrate Metabolism
3D Structures of Alcohol dehydrogenase
Updated on 16-July-2013
ADH I
3jv7 – RrADH I – Rhodococcus rubber
2vna - hADH I catalytic domain - human
2hcy – yADH I – yeast
4eex – LlADH I – Lactococcus lactis
4eez – LlADH I (mutant)
ADH I binary complex
1u3t – hADH I α chain + inhibitor
1hsz, 1hdz, 3hud - hADH I β chain + NAD
1u3w - hADH I γ chain + inhibitor
1ht0 - hADH I γ chain (mutant) + NAD
ADH I ternary complex
2xaa – RrADH I + NAD + alcohol
3fx4 – pADH I + NADP + inhibitor – pig
2w98, 2w4q – hADH I catalytic domain + NADP + inhibitor
1hso - hADH I α chain + NAD + pyrazole derivative
1hdx - hADH I β chain + NAD + alcohol
1u3u, 1u3v - hADH I β chain + inhibitor
1deh, 1hdy - hADH I β chain + NAD + pyrazole derivative
1htb - hADH I β3 chain + NAD + pyrazole derivative
ADH II
3owo – ZmADH II iron-dependent – Zymomonas mobilis
ADH II binary complex
3ox4 - ZmADH II iron-dependent + NAD
3cos - hADH II + NAD + Zn
1e3e – mADH II + NADH – mouse
1e3l - mADH II (mutant) + NADH
1e3i - mADH II + NADH + inhibitor
ADH III
1m6h, 1m6w, 1teh - hADH III χ chain
2fze - hADH III χ chain + ADP-ribose
2fzw - hADH III χ chain + NAD
1mc5 – hADH III χ chain + glutathione + NADH
1ma0 - hADH III χ chain + dodecanoic acid + NAD
3qj5 - hADH III χ chain + inhibitor + NAD
4dl9, 4dlb – tADH III + NAD – tomato
4dla – tADH III
ADH IV
1ye3, 8adh, 5adh - hoADH IV e chain – horse
1qlj - hoADH IV e chain (mutant)
3iv7 – ADH IV – Corynebacterium glutamicum
ADH IV binary complex
2jhf, 2jhg, 1het, 1heu, 1hf3, 1ee2, 2oxi, 2ohx, 6adh - hoADH IV e chain + NAD
1adb, 1adc, 1adf, 1adg, 7adh - hoADH IV e chain + NAD derivative
1mgo, 1ju9, 1qlh, 1a72 - hoADH IV e chain (mutant) + NAD
1d1s, 1agn – hADH IV σ chain + NAD
1d1t - hADH IV σ chain (mutant) + NAD
ADH IV ternary complex
3oq6, 1qv6, 1qv7, 1a71, 1axe, 1axg – hoADH IV e chain (mutant) + NAD + alcohol
4dwv, 4dxh - hoADH IV e chain + NAD + alcohol
1p1r, 1ldy, 1lde - hoADH IV e chain + NADH + formamide derivative
1n92 - hoADH IV e chain + NAD + pyrazole derivative
1bto, 3bto - hoADH IV e chain + NADH + butylthiolane derivative
1n8k - hoADH IV e chain (mutant) + NAD + pyrazole
1mg0, 1hld - hoADH IV e chain + NAD + alcohol
ADH
1a4u – SlADH – Scaptodrosophila lebanonensis
3my7 – ADH ACDH domain – Vibrio parahaemolyticus
3meq – ADH – Brucella suis
3l4p – ADH – Desulfovibrio gigas
1jvb - SsADH – Sulfolobus solfataricus
3i4c, 1nto, 1nvg – SsADH (mutant)
3goh – ADH – Shewanella oneidensis
3gaz – ADH residues 2-334 – Novosphingobium aromaticivorans
2eih – ADH – Thermus thermophilus
1rjw – GsADH – Geobacillus stearothermophilus
1vj0, 1vhd – TmADH -Thermotoga maritima
2eer – ADH – Sulfolobus tokodaii
3uog – ADH – Sinorhizobium meliloti
ADH binary complex
3l77, 3tn7 – ADH short-chain + NADP – Thermococcus sibiricus
1h2b – ADH + NAD – Aeropyrum pernix
1f8f – Benzyl-ADH + NAD – Acinetobacter calcoaceticus
1o2d - TmADH + NADP
1b16, 1b14, 1b15 - SlADH + NAD derivative
1cdo – ADH + NAD - cod
1rhc – ADH F420-dependent +F420-acetone – Methanoculleus thermophilus
1agn – hADH (sigma) +NAD
3pii – GsADH + butyramide
3rj5, 3rj9 – SlADH (mutant) + NAD
3s1l – ADH + Zn – Ralstonia eutropha
ADH ternary complex
1mg5 – ADH + NADH + acetate – Drosophila melanogaster
1r37 – SsADH + NAD + alcohol
1sby – SlADH + NAD + alcohol
1b2l - SlADH + NAD + cyclohexanone
1llu - ADH + NAD + alcohol – Pseudomonas aeruginosa
3cv7 – pADH + NAD + NAP
3rf7 – SoADH + NAD + Fe + Ni
NADP-dependent ADH
1ped - CbADH – Clostridium beijerinckii
2b83, 1jqb – CbADH (mutant)
2nvb - TbADH (mutant) – Thermoanaerobacter brockii
3ftn, 3fpc, 3fpl, 3fsr – ADH chimera
1y9a - EhADH – Entamoeba histolytica
2oui – EhADH (mutant)
1p0c – RpADH8 – Rana perezi
4gac - mADH
NADP-dependent ADH binary complex
1kev – CbADH + NADPH
1bxz – CbADH catalytic domain + alcohol
1ykf – TbADH + NADP
3h4g – pADH + NADP
1p0f – RpADH + NADP
R-specific ADH
1nxq - LbRADH – Lactobacillus brevis
1zk2, 1zk3 - LbRADH (mutant)
1zjy, 1zjz, 1zk0, 1zk1 – LbRADH (mutant) + NADH + alcohol
1zk4 - LbRADH (mutant) + NADH + acetophenone
Specific alcohol ADH
2cf5, 2cf6 – Cinnamyl-ADH – Arabidopsis thaliana
1piw, 1q1n, 1ps0 – Cinnamyl-yADH
3two - Cinnamyl-ADH + NADP – Helicobacter pylori
1m2w – Mannitol-ADH – Pseudomonas fluorescens
1w6s – Methanol-ADH – Methylobacterium extorquens
1yqx – Sinapyl-aADH II – aspen
1yqd – Sinapyl-aADH II + NADP
1bdb – Biphenyl dihydrodiol-ADH + NAD - Pseudomonas
Quinohemoprotein ADH
1kv9, 1yiq – PpQADH II + PQQ + heme – Pseudomonas putida
1kb0 - QADH I + PQQ + heme – Comamonas testosteroni
Hydroxyacyl-CoA dehydrogenase
Short chain HADH
1so8 – hSHCDH II – human
3rqs - hSHCDH
1f14 - hSHCDH (mutant)
Short chain HADH binary complex
1f12 - hSHCDH (mutant) + hydroxybutyryl-CoA
1f17, 1lsj, 1lso - hSHCDH (mutant) + NAD
1zbq - hSHCDH IV + NAD
1e3s - rSHCDH + NAD – rat
Short chain HADH ternary complex
1u7t - hSHCDH II + inhibitor + NAD
1f0y - hSHCDH + acetoacetyl-CoA + NAD
1il0, 1m75, 1m76 - hSHCDH (mutant) + acetoacetyl-CoA + NAD
1e3w - rSHCDH + 3-keto-butyrate + NAD
1e6w - rSHCDH + estradiol + NAD
Unspecified HADH
1uay - HADH II – Thermus thermophilus
1zej, 3ctv - HADH – Archaeoglobus fulgidus
1zcj - rHADH
2x58 - rHADH + CoA
2et6 – HADH (mutant) – Candida tropicalis
References
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol dehydrogenase from Human (Homo sapiens), different isozymes. SCOP. 2009. 1 March 2010 < http://scop.berkeley.edu/data/scop.b.d.c.b.b.c.html>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Bille V, Remacle J. Simple-kinetic descriptions of alcohol dehydrogenase after immobilization on tresyl-chloride-activated agarose. Eur J Biochem. 1986 Oct 15;160(2):343-8. PMID:3769934
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Blomstrand R, Ostling-Wintzell H, Lof A, McMartin K, Tolf BR, Hedstrom KG. Pyrazoles as inhibitors of alcohol oxidation and as important tools in alcohol research: an approach to therapy against methanol poisoning. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3499-503. PMID:115004
- ↑ Alcohol Dehydrogenase. Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Alcohol Dehydrogenase.Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Goihberg E, Dym O, Tel-Or S, Levin I, Peretz M, Burstein Y. A single proline substitution is critical for the thermostabilization of Clostridium beijerinckii alcohol dehydrogenase. Proteins. 2007 Jan 1;66(1):196-204. PMID:17063493 doi:10.1002/prot.21170
- ↑ Goihberg E, Dym O, Tel-Or S, Shimon L, Frolow F, Peretz M, Burstein Y. Thermal stabilization of the protozoan Entamoeba histolytica alcohol dehydrogenase by a single proline substitution. Proteins. 2008 Feb 7;. PMID:18260103 doi:10.1002/prot.21946
- ↑ Goihberg E, Peretz M, Tel-Or S, Dym O, Shimon L, Frolow F, Burstein Y. Biochemical and Structural Properties of Chimeras Constructed by Exchange of Cofactor-Binding Domains in Alcohol Dehydrogenases from Thermophilic and Mesophilic Microorganisms. Biochemistry. 2010 Feb 9. PMID:20102159 doi:10.1021/bi901730x
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Joel L. Sussman, David Birrer