2iy0
From Proteopedia
(New page: 200px<br /> <applet load="2iy0" size="450" color="white" frame="true" align="right" spinBox="true" caption="2iy0, resolution 2.77Å" /> '''SENP1 (MUTANT) SUMO...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:2iy0.gif|left|200px]]<br /> | + | [[Image:2iy0.gif|left|200px]]<br /><applet load="2iy0" size="350" color="white" frame="true" align="right" spinBox="true" |
| - | <applet load="2iy0" size=" | + | |
caption="2iy0, resolution 2.77Å" /> | caption="2iy0, resolution 2.77Å" /> | ||
'''SENP1 (MUTANT) SUMO1 RANGAP'''<br /> | '''SENP1 (MUTANT) SUMO1 RANGAP'''<br /> | ||
==Overview== | ==Overview== | ||
| - | Small ubiquitin-like modifier (SUMO)-specific protease SENP1 processes | + | Small ubiquitin-like modifier (SUMO)-specific protease SENP1 processes SUMO-1, SUMO-2 and SUMO-3 to mature forms and deconjugates them from modified proteins. To establish the proteolytic mechanism, we determined structures of catalytically inactive SENP1 bound to SUMO-1-modified RanGAP1 and to unprocessed SUMO-1. In each case, the scissile peptide bond is kinked at a right angle to the C-terminal tail of SUMO-1 and has the cis configuration of the amide nitrogens. SENP1 preferentially processes SUMO-1 over SUMO-2, but binding thermodynamics of full-length SUMO-1 and SUMO-2 to SENP1 and K(m) values for processing are very similar. However, k(cat) values differ by 50-fold. Thus, discrimination between unprocessed SUMO-1 and SUMO-2 by SENP1 is based on a catalytic step rather than substrate binding and is likely to reflect differences in the ability of SENP1 to correctly orientate the scissile bonds in SUMO-1 and SUMO-2. |
==Disease== | ==Disease== | ||
| Line 11: | Line 10: | ||
==About this Structure== | ==About this Structure== | ||
| - | 2IY0 is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http:// | + | 2IY0 is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2IY0 OCA]. |
==Reference== | ==Reference== | ||
| Line 18: | Line 17: | ||
[[Category: Protein complex]] | [[Category: Protein complex]] | ||
[[Category: Dong, C.]] | [[Category: Dong, C.]] | ||
| - | [[Category: Naismith, J | + | [[Category: Naismith, J H.]] |
[[Category: Shen, L.]] | [[Category: Shen, L.]] | ||
[[Category: gtpase activation]] | [[Category: gtpase activation]] | ||
| Line 32: | Line 31: | ||
[[Category: ubl conjugation pathway]] | [[Category: ubl conjugation pathway]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 17:57:16 2008'' |
Revision as of 15:57, 21 February 2008
|
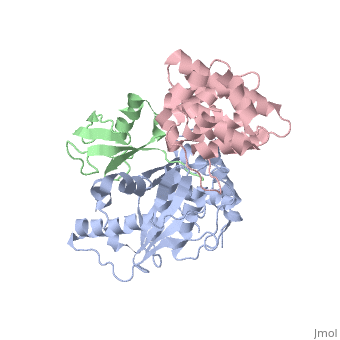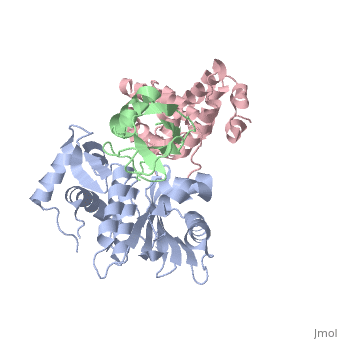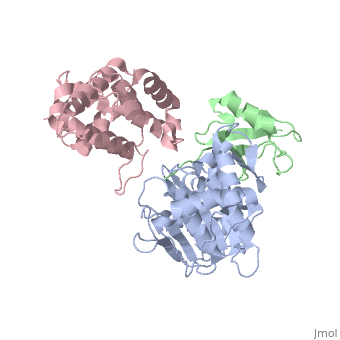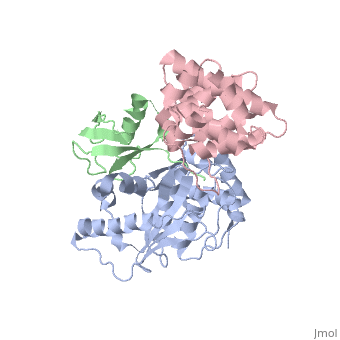
SENP1 (MUTANT) SUMO1 RANGAP
Contents |
Overview
Small ubiquitin-like modifier (SUMO)-specific protease SENP1 processes SUMO-1, SUMO-2 and SUMO-3 to mature forms and deconjugates them from modified proteins. To establish the proteolytic mechanism, we determined structures of catalytically inactive SENP1 bound to SUMO-1-modified RanGAP1 and to unprocessed SUMO-1. In each case, the scissile peptide bond is kinked at a right angle to the C-terminal tail of SUMO-1 and has the cis configuration of the amide nitrogens. SENP1 preferentially processes SUMO-1 over SUMO-2, but binding thermodynamics of full-length SUMO-1 and SUMO-2 to SENP1 and K(m) values for processing are very similar. However, k(cat) values differ by 50-fold. Thus, discrimination between unprocessed SUMO-1 and SUMO-2 by SENP1 is based on a catalytic step rather than substrate binding and is likely to reflect differences in the ability of SENP1 to correctly orientate the scissile bonds in SUMO-1 and SUMO-2.
Disease
Known diseases associated with this structure: Blood group, Cad system OMIM:[111730], Blood group, Sd system OMIM:[111730], Orofacial cleft 10 OMIM:[601912]
About this Structure
2IY0 is a Protein complex structure of sequences from Homo sapiens. Full crystallographic information is available from OCA.
Reference
SUMO protease SENP1 induces isomerization of the scissile peptide bond., Shen L, Tatham MH, Dong C, Zagorska A, Naismith JH, Hay RT, Nat Struct Mol Biol. 2006 Dec;13(12):1069-77. Epub 2006 Nov 12. PMID:17099698
Page seeded by OCA on Thu Feb 21 17:57:16 2008
Categories: Homo sapiens | Protein complex | Dong, C. | Naismith, J H. | Shen, L. | Gtpase activation | Hydrolase | Hydrolase/activator complex ubl conjugation | Leucine-rich repeat | Nuclear protein | Phosphorylation | Protease | Protein protein complex | Thiol protease | Ubiquitin | Ubl conjugation pathway