We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 126
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='4dki' size= | + | <StructureSection load='4dki' size=525 side=right scene='4dki'/Com_view/1'> |
== Background == | == Background == | ||
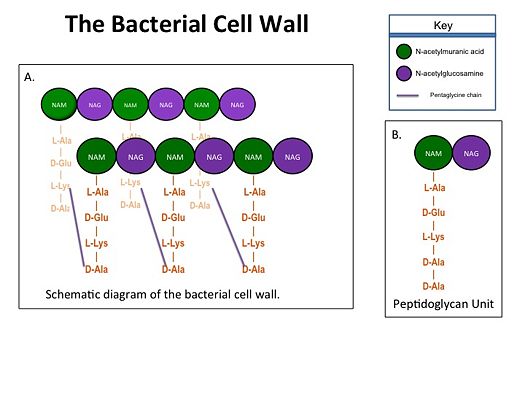
| - | Transpeptidases (TP), also known as penicillin-binding proteins (PBP), catalyze the cross-linking of peptidoglycan polymers during bacterial cell wall synthesis. The natural transpeptidase substrate is the D-Ala-D-Ala peptidoglycan side chain terminus. Beta-lactam (β-lactam) antibiotics, which include penicillins, cephalosporins and carbapenems, bind and irreversibly inhibit transpeptidases by mimicking the D-Ala-D-Ala substrate, resulting in the inhibition of cell wall synthesis and ultimately bacterial cell growth. [[Image:Cell Wall 7 30 2013.jpg]]Overuse and misuse of β-lactams has led to the generation of methicillin- resistant Staphylococcus aureus (MRSA) isolates that have acquired an alternative transpeptidase, PBP2a, which is neither bound nor inhibited by β- lactams. MRSA isolates are resistant to all β-lactams, and are often only susceptible to “last resort antibiotics”, such as vancomycin. Recently, two cephalosporins - ceftobiprole and ceftaroline - that bind and inhibit PBP2a have been developed. | + | Transpeptidases (TP), also known as penicillin-binding proteins (PBP), catalyze the cross-linking of peptidoglycan polymers during bacterial cell wall synthesis. The natural transpeptidase substrate is the D-Ala-D-Ala peptidoglycan side chain terminus. Beta-lactam (β-lactam) antibiotics, which include penicillins, cephalosporins and carbapenems, bind and irreversibly inhibit transpeptidases by mimicking the D-Ala-D-Ala substrate, resulting in the inhibition of cell wall synthesis and ultimately bacterial cell growth. [[Image:Cell Wall 7 30 2013.jpg|thumb|alt= Alt text| Figure 1. A.Bacterial Cell Wall B.Peptidoglycan with D-Ala-D-Ala substrate |525px]] Overuse and misuse of β-lactams has led to the generation of methicillin- resistant Staphylococcus aureus (MRSA) isolates that have acquired an alternative transpeptidase, PBP2a, which is neither bound nor inhibited by β- lactams. MRSA isolates are resistant to all β-lactams, and are often only susceptible to “last resort antibiotics”, such as vancomycin. Recently, two cephalosporins - ceftobiprole and ceftaroline - that bind and inhibit PBP2a have been developed. |
<scene name='37/372726/Pbp2a_with_residues/1'>PBP2a and active site residues</scene> | <scene name='37/372726/Pbp2a_with_residues/1'>PBP2a and active site residues</scene> | ||
Revision as of 18:21, 30 July 2013
| |||||||||||