PPAR-gamma
From Proteopedia
| Line 1: | Line 1: | ||
| - | < | + | <StructureSection load='3et3' size='450' side='right' scene='' caption=''> |
== Introduction == | == Introduction == | ||
| Line 9: | Line 9: | ||
PPARγ is composed of the ligand-independent activation domain (AF-1 region and A/B-domain), a DNA-binding domain (DBD) (C-domain), a hinge region (D-domain), and a ligand-dependent ligand-binding domain (LBD) (E/F-domain and AF-2 region) [5]. The two PPARγ isoforms, PPARγ1 and PPARγ2, differ by only 30 amino acids at the N-terminal end. These added amino acids on PPARγ2 result in increased potency and adipose-selectivity, which makes this protein a key player of adipocyte differentiation [3]. | PPARγ is composed of the ligand-independent activation domain (AF-1 region and A/B-domain), a DNA-binding domain (DBD) (C-domain), a hinge region (D-domain), and a ligand-dependent ligand-binding domain (LBD) (E/F-domain and AF-2 region) [5]. The two PPARγ isoforms, PPARγ1 and PPARγ2, differ by only 30 amino acids at the N-terminal end. These added amino acids on PPARγ2 result in increased potency and adipose-selectivity, which makes this protein a key player of adipocyte differentiation [3]. | ||
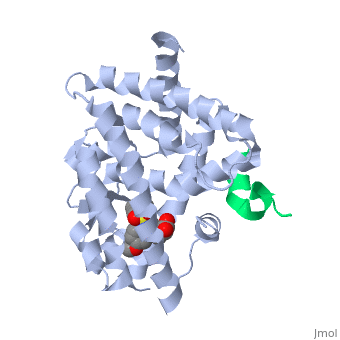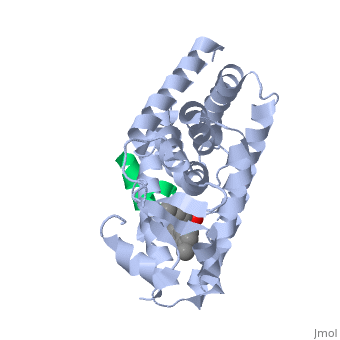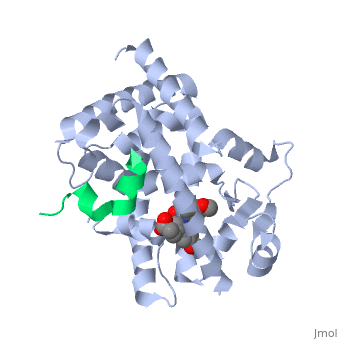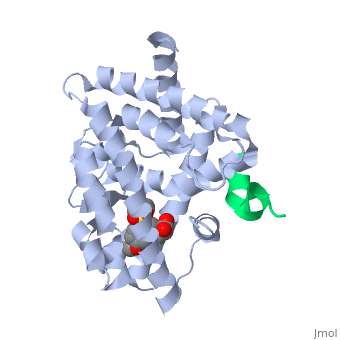
The <scene name='PPAR-gamma/Lbd/2' target='2'>ligand binding domain</scene> is composed of 13 α helices and 4 short β strands [1]. It has a T-shaped binding pocket with a volume of ~1440 Å3 [1, 6], which is larger than that of most nuclear receptors [7], allowing for interactions with a variety of ligands [8]. The PPARγ LBD is folded into a helical sandwich to provide a binding site for ligands. It is located at the C-terminal end of PPARγ and is composed of about 250 amino acids [5]. Activation by full agonists occurs through hydrogen bond interactions between the S289, H323, Y473, and H449 residues of the PPARγ-LBD [7] and polar functional groups on the ligand which are typically carbonyl or carboxyl oxygen atoms. Agonist binding results in a conformational change of the LBD AF-2 region, which is necessary for coactivator recruitment. This change can either be dramatic or subtle [1], which leads to stabilization of a charge clamp between helices H3 and H12 [9] to aid in associations with the LXXLL (L, leucine; X, any amino acid) motif of the coactivator [1, 10]. Ligand binding of PPARγ is regulated by communication between the N-terminal A/B domain, which is adjacent to the DBD, and the carboxyl-terminal LBD [11]. | The <scene name='PPAR-gamma/Lbd/2' target='2'>ligand binding domain</scene> is composed of 13 α helices and 4 short β strands [1]. It has a T-shaped binding pocket with a volume of ~1440 Å3 [1, 6], which is larger than that of most nuclear receptors [7], allowing for interactions with a variety of ligands [8]. The PPARγ LBD is folded into a helical sandwich to provide a binding site for ligands. It is located at the C-terminal end of PPARγ and is composed of about 250 amino acids [5]. Activation by full agonists occurs through hydrogen bond interactions between the S289, H323, Y473, and H449 residues of the PPARγ-LBD [7] and polar functional groups on the ligand which are typically carbonyl or carboxyl oxygen atoms. Agonist binding results in a conformational change of the LBD AF-2 region, which is necessary for coactivator recruitment. This change can either be dramatic or subtle [1], which leads to stabilization of a charge clamp between helices H3 and H12 [9] to aid in associations with the LXXLL (L, leucine; X, any amino acid) motif of the coactivator [1, 10]. Ligand binding of PPARγ is regulated by communication between the N-terminal A/B domain, which is adjacent to the DBD, and the carboxyl-terminal LBD [11]. | ||
| - | |||
| - | <applet load='2f4b' size='300' frame='true' align='left' caption='PPARγ Ligand Binding Domain complex with agonist [[2f4b]]' name='2' /> | ||
== Ligand Activity == | == Ligand Activity == | ||
| Line 33: | Line 31: | ||
PPARγ is found in high levels in colonic epithelial cells. The role of PPARγ in these cells may be related to regulation of immune response and colon inflammation [12]. The onset of Inflammatory Bowel Disease is thought to be caused by inflammatory cytokines present in the colon [12]. In patients with ulcerative colitis, colonic epithelial cells displayed impaired expression of PPARγ, an important mediator of aminosalicylate activities in Inflammatory Bowel Diseases [13]. TZD ligands could be implemented to reduce colonic inflammation [12]. Agonists have also been used in the treatment of colitis and psoriasis by inhibiting the inflammatory response of the epithelium and reducing cytokine production [8]. PPARγ inhibits activity of nuclear factor NFκB, which is higher in active ulcerative colitis patients [15]. | PPARγ is found in high levels in colonic epithelial cells. The role of PPARγ in these cells may be related to regulation of immune response and colon inflammation [12]. The onset of Inflammatory Bowel Disease is thought to be caused by inflammatory cytokines present in the colon [12]. In patients with ulcerative colitis, colonic epithelial cells displayed impaired expression of PPARγ, an important mediator of aminosalicylate activities in Inflammatory Bowel Diseases [13]. TZD ligands could be implemented to reduce colonic inflammation [12]. Agonists have also been used in the treatment of colitis and psoriasis by inhibiting the inflammatory response of the epithelium and reducing cytokine production [8]. PPARγ inhibits activity of nuclear factor NFκB, which is higher in active ulcerative colitis patients [15]. | ||
PPARγ could also be implemented in the treatment of other chronic inflammation-related diseases. Immunomodulatory effects have been found with PPARγ agonists [16]. Rosiglitazone alongside adiponectin reduces renal disease, atherosclerosis, and production of autoantibodies, all of which are characteristic of the inflammatory autoimmune disease Systemic Lupus Erythematosus (SLE) [16]. PPARγ ligands hold potential as cancer treatments [11] due to their ability to inhibit angiogenesis, the process required for the growth and metastasis of solid tumors [8]. PPARγ activators have pro-differentiation and anti-proliferation effects [3]. TZDs have also been shown to inhibit proliferation of human breast, prostate, and colon cancer cells [8]. | PPARγ could also be implemented in the treatment of other chronic inflammation-related diseases. Immunomodulatory effects have been found with PPARγ agonists [16]. Rosiglitazone alongside adiponectin reduces renal disease, atherosclerosis, and production of autoantibodies, all of which are characteristic of the inflammatory autoimmune disease Systemic Lupus Erythematosus (SLE) [16]. PPARγ ligands hold potential as cancer treatments [11] due to their ability to inhibit angiogenesis, the process required for the growth and metastasis of solid tumors [8]. PPARγ activators have pro-differentiation and anti-proliferation effects [3]. TZDs have also been shown to inhibit proliferation of human breast, prostate, and colon cancer cells [8]. | ||
| - | + | </StructureSection> | |
| + | __NOTOC__ | ||
==3D structures of PPAR== | ==3D structures of PPAR== | ||
Revision as of 12:20, 1 August 2013
| |||||||||||
3D structures of PPAR
Peroxisome Proliferator-Activated Receptors
Additional Resources
For additional information See: Diabetes
For additional information See: Regulation of Gene Expression
For additional information See: Peroxisome Proliferator-Activated Receptors
References
[1] Gampe Jr RT, Montana VG, Lambert MH, et al. Asymmetry in the PPARγ/RXRα crystal structure reveals the molecular basis of heterodimerization among nuclear receptors (2000) Molecular Cell, 15(9), pp.545-555.
[2] Zieleniak A, Wójcik M, Woźniak LA. Structure and physiology functions of the human peroxisome proliferator-activated receptor γ (2008) Arch. Immunol. Ther. Exp., 56 (5), pp. 331-345.
[3] Tontonoz P, Spiegelman BM. Fat and Beyond: The Diverse Biology of PPARγ (2008) Annu. Rev. Biochem., 77, pp. 289-312.
[4] Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism (1999) Endocrine Reviews, 20 (5), pp. 649-688.
[5] Lewis SN, Bassaganya-Riera J, Bevan DR. Virtual Screening as a Technique for PPAR Modulatory Discovery (2010) PPAR Research, 2010, pp. 861238.
[6] Itoh T, Fairall L, Amin K, et al. Structural basis for the activation of PPARγ by oxidized fatty acids (2008) Nature Structural and Molecular Biology, 15 (9), pp.924-931.
[7] Pochetti G, Godio C, Mitro N, et al. Insights into the mechanism of partial agonism: crystal structures of the peroxisome proliferator-activated receptor γ ligand-binding domain in the complex with two enantiomeric ligands (2007) Journal of Biological Chemistry, 282 (23), pp.17314-17324.
[8] Murphy GJ, Holder JC. PPAR-γ agonists: Therapeutic role in diabetes, inflammation and cancer (2000) Trends in Pharmacological Sciences, 21 (12), pp. 469-474.
[9] Xu HE, Stanley TB, Montana VG, et al. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARα (2002) Nature, 415 (6873), pp.813-817.
[10] Kallenberger BC, Love, JD, Chatterjee VKK, Schwabe JWR. A dynamic mechanism of nuclear receptor activation and its perturbation in a human disease (2003) Nature, 10 (2), pp.136-140.
[11] Shao D, Rangwala SM, Bailey ST, Krakow SA, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPARγ (1998) Nature, 396, pp. 377-380.
[12] Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A Novel therapy for colitis utilizing PPAR-γ ligands to inhibit the epithelial inflammatory response (1999) J Clin Invest., 104(4), pp. 383-389.
[13] Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M, Desreumaux P. PPARγ as a new therapeutic target in inflammatory bowel disease (2006) International Journal of Gastroenterology and Hepatology, 55 (9), pp.1341-1349.
[14] McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators (2002) Cell, 108 (4), pp. 465-474.
[15] Sartor, RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis (2006) Nature, 3(7), pp. 390-407.
[16] Aprahamian T, Bonegio RG, Richez C, Yasuda K, Chiang L, Sato K, Walsh K, Rifkin IR. The Peroxisome Proliferator-Activated Receptor γ Agonist Rosiglitazone Ameliorates Murine Lupus by Induction of Adiponectin (2009) the Journal of Immunology, 182, pp. 340 -346.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Lera Brannan, David Canner, Alexander Berchansky