Peptidoglycan transpeptidases (TP), also known as penicillin-binding proteins (PBP), are essential for bacterial cell wall synthesis and catalyze the cross-linking of peptidoglycan polymers during bacterial cell wall synthesis. Beta-lactam (β-lactam) antibiotics, which include the penicillins, cephalosporins, carbapenems, and the monobactam aztreonam (Figure 1); bind and irreversibly inhibit the active site of TP. The overuse and misuse of β-lactam antibiotics has led to strains of Staphylococcus aureus (S. aureus) that are resistant to almost all currently available β-lactams and are often only susceptible to so-called “last resort antibiotics”, such as vancomycin.
Cell Wall Structure
The bacterial cell wall is crucial for maintaining the structural integrity of bacteria and protects bacteria from osmotic stress and toxic compounds. The cell wall is composed of peptidoglycan (Figure 2 ) and in Gram positive bacterial species (e.g., S. aureus) is many layers thick, while in Gram negative bacterial species (e.g., Escherichia coli) is only a few layers thick. The difference in the number of peptidoglycan layers accounts for the differential staining of these two groups of organisms. . Peptidoglycan consists of a carbohydrate portion: alternating residues of N-acetylmuranic Acid (NAM) and N-acetylglucosamine (NAG) that polymerize to form long chains, and a protein portion: a pentapeptide chain that terminates with two D-alanine (D-Ala) residues. The pentapeptide chains are covalently bound to each NAM residue Rows of peptidoglycan are cross-linked together with pentaglycine chains to form a "mesh-like" structure. This cross-linking reaction is catalyzed by TPs.
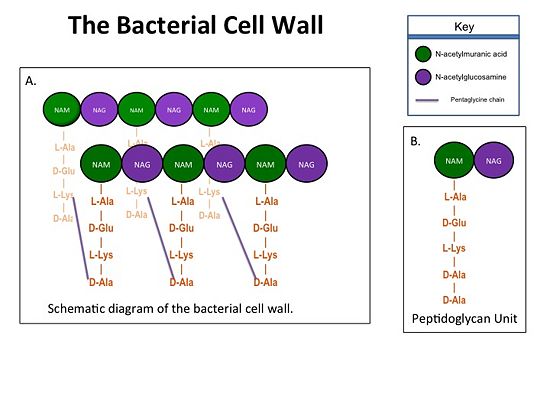
Figure 1. A.Bacterial Cell Wall B.Peptidoglycan with D-Ala-D-Ala substrate
Structure of a Resistant Transpeptidase
Methicillin resistant Staphylococcus aureus (MRSA) is resistant to all β-lactams because it acquires an alternative PBP, PBP2a, that is not bound or inhibited by any β-lactams. PBP2a is composed of two domains: a non-penicillin binding domain and a transpeptidase binding domain . The NBP domain of PBP2a is anchored in the cell membrane, while the TP domain “sits” in the periplasm with its active site facing the inner surface of the cell wall. The active site contains which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links.
Catalytic Mechanism of Action of Transpeptidases
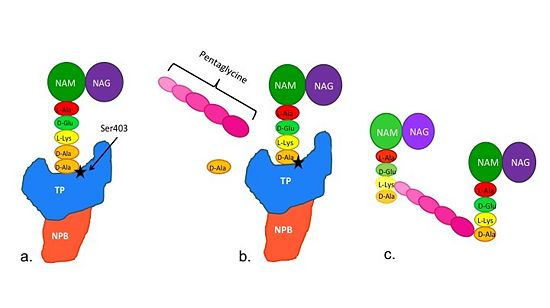
Figure 2. Schematic diagram illustrating the mechanism of action of PBP2a
Note: Schematic is above these statements:
(a)The D-Ala-D-Ala side-chain substrate accesses the TP active site.
(b) The active site serine residue nucleophilically attacks the peptide bond between the terminal D-Ala residues. The terminal D-Ala residue exits the active site, and the remaining D-Ala residue forms a covalent bond with the active site serine residue to form an acyl-TP complex.
(c) Subsequently, a pentaglycine chain enters the TP active site through nucleophillic attack forms a covalent bond with the D-Ala residue formerly bound to the active site serine residue. As a result, the TP active site serine residue is regenerated. The entire process takes approximately 4 milliseconds.
Mechanism of Action of β-Lactam Antibiotics
The β-lactam antibiotics inhibit bacterial growth by irreversibly inhibiting TPs and, therefore, bacterial cell wall synthesis. Specifically, β-lactams are molecular mimics of a portion of the peptidoglycan polymer, namely the D-Ala-D-Ala moiety, which is the normal TP enzymatic substrate (Figure 4). As a result, bacterial TP enzymes are "tricked" into reacting with β-lactams. Additionally, the β-lactams are very reactive molecules due to their β-lactam ring, and readily react with the TP active site serine residue and sterically block the active site preventing the entry of nucleophiles [1] that regenerate the active site serine residue such as the pentaglycine chain or water.
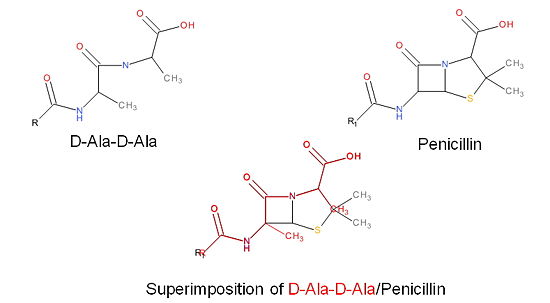
Figure 3. Mechanism of action of β-lactams. A. Structure of a β-lactam (penicillin) showing the amide, carboxyl, and β-lactam ring groups β-lactam ring groups. B. Structure of the D-Ala-D-Ala substrate. C. Overlay of the D-Ala-D-Ala substrate in red with penicillin demonstrating molecular mimicry.
Structure of PBP2a, a B-lactam Resistant Transpeptidase
Isolates of methicillin-resistant S. aureus (MRSA) are resistant to almost all currently available B-lactam because they have acquired an alternative PBP, PBP2a(encoded by the mecA gene), that is neither bound nor inhibited by B-lactams. PBP2a is composed of two domains:a a non-penicillin binding domain and a transpeptidase binding domain non-penicillin binding domain (NPB) and a TP-binding domain. The NPB domain of PBP2a is anchored in the cell membrane, while the TP domain resides in the periplasm with its active site facing the inner surface of the cell wall. The active site contains a serine residue at position 403 (Ser403) which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links.
B-Lactams that Inhibit PBP2a
MRSA becomes resistant to almost all β-lactams by acquiring an alternative TP, PBP2a, that is
neither bound nor inhibited by β-lactams. Recently, two cephalosporins –
and
ceftaroline – that have anti-MRSA activity have been developed. Ceftobiprole is able to
inhibit PBP2a because additional chemical groups at the
position of the ceftobiprole are able to interact with additional amino acid
residues in PBP2a; specifically
. As such, ceftobiprole is (shown as colors of the atom types (CPK))is able to more efficiently react with and therefore inhibit the activity of PBP2a.



