We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 795
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
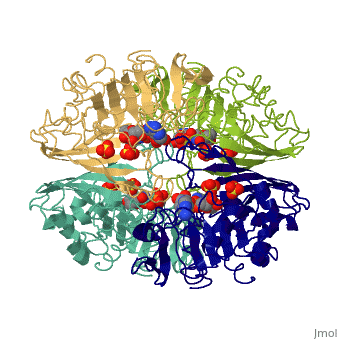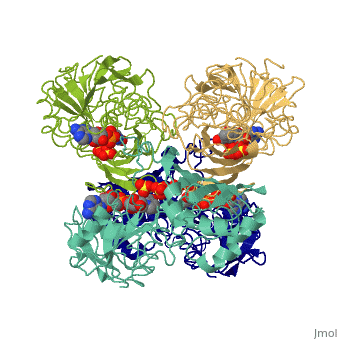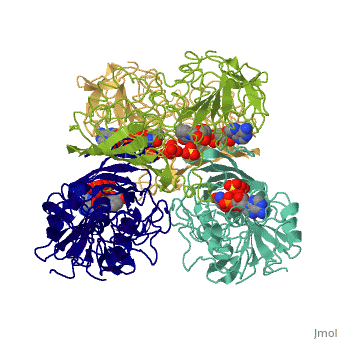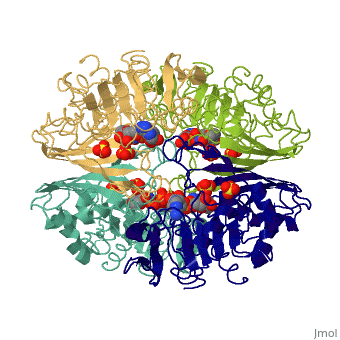
<Structure load='3gpd' size='500' frame='true' align='right' caption='Glyceraldehyde-3-phosphate dehydrogenase' scene='Insert optional scene name here' /> Glyceraldehyde 3-phosphate dehydrogenase, or G-3-P dehydrogenase, is an enzyme that plays a significant role in metabolism. It catalyzes a step in glycolysis in which glyceraldehyde 3-phosphate is converted to 3-phospho-D-glyceroyl phosphate. G-3-P dehydrogenase is a tetramer protein made up of four subunits. The <scene name='56/563207/Secondary_structure/1'>secondary structure</scene> of G-3-P dehydrogenase consists of alpha helices which are displayed in purple and beta sheets which are displayed in green. | <Structure load='3gpd' size='500' frame='true' align='right' caption='Glyceraldehyde-3-phosphate dehydrogenase' scene='Insert optional scene name here' /> Glyceraldehyde 3-phosphate dehydrogenase, or G-3-P dehydrogenase, is an enzyme that plays a significant role in metabolism. It catalyzes a step in glycolysis in which glyceraldehyde 3-phosphate is converted to 3-phospho-D-glyceroyl phosphate. G-3-P dehydrogenase is a tetramer protein made up of four subunits. The <scene name='56/563207/Secondary_structure/1'>secondary structure</scene> of G-3-P dehydrogenase consists of alpha helices which are displayed in purple and beta sheets which are displayed in green. | ||
| + | |||
| + | ==Hydrogen Bonds== | ||
| + | |||
| + | The <scene name='56/563207/Hydrogen_bonding/1'>hydrogen bonding</scene> of the backbone is displayed in orange. There are no disulfide bonds present in this enzyme. | ||
Revision as of 01:53, 15 October 2013
| This Sandbox is Reserved from Oct 10, 2013, through May 20, 2014 for use in the course "CHEM 410 Biochemistry 1 and 2" taught by Hanna Tims at the Messiah College. This reservation includes Sandbox Reserved 780 through Sandbox Reserved 807. |
To get started:
More help: Help:Editing |
Introduction and General Structure
|
Hydrogen Bonds
The of the backbone is displayed in orange. There are no disulfide bonds present in this enzyme.