This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Single stranded binding protein
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
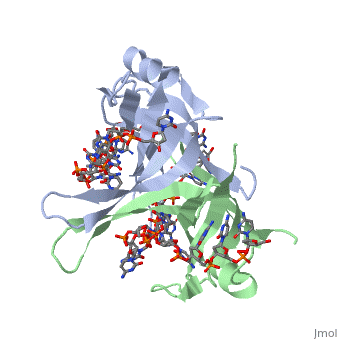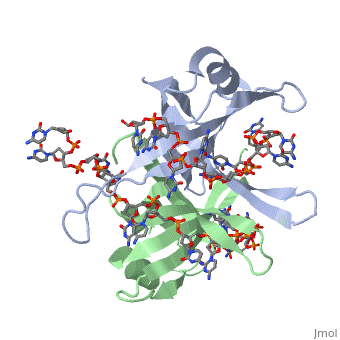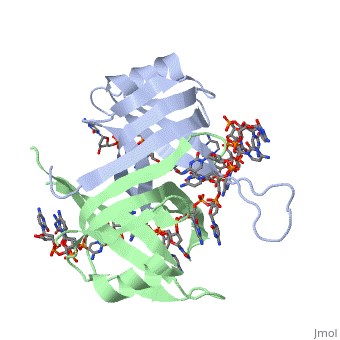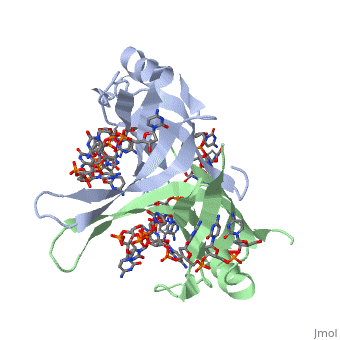
<StructureSection load='1eyg' size='500' side='right' frame='true' caption='Structure of Single Stranded DNA-Binding Protein bound to ssDNA (PDB entry [[1eyg]])' scene=''> | <StructureSection load='1eyg' size='500' side='right' frame='true' caption='Structure of Single Stranded DNA-Binding Protein bound to ssDNA (PDB entry [[1eyg]])' scene=''> | ||
| - | + | ==Introduction== | |
The single stranded DNA binding protein (SSB) of E. coli plays an important role in three aspects of DNA metabolism – namely in replication, repair and recombination. During DNA replication, SSB molecules bind to the newly separated DNA strands, keeping the strands separated by holding them in place so that each strand can serve as a template. | The single stranded DNA binding protein (SSB) of E. coli plays an important role in three aspects of DNA metabolism – namely in replication, repair and recombination. During DNA replication, SSB molecules bind to the newly separated DNA strands, keeping the strands separated by holding them in place so that each strand can serve as a template. | ||
| - | + | ==Structure== | |
The structure of SSB consists of a homotetramer that has a DNA binding domain that binds to a single strand of DNA. | The structure of SSB consists of a homotetramer that has a DNA binding domain that binds to a single strand of DNA. | ||
| Line 18: | Line 18: | ||
</StructureSection> | </StructureSection> | ||
| - | + | ==See Also== | |
Revision as of 16:58, 30 October 2013
Contents |
Sandbox Single Stranded DNA-Binding Protein (SSB)
| |||||||||||
Binding Interactions in the Active Site
| |||||||||||
See Also
References
PMID: 2087220
Proteopedia Page Contributors and Editors (what is this?)
Refayat Ahsen, Rachel Craig, Michal Harel, Alexander Berchansky