We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ku protein
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
=== Ku Ring === | === Ku Ring === | ||
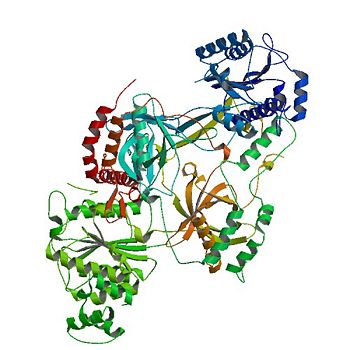
| - | The <scene name='56/567269/Ku_ring/1'>Ku Ring</scene> is composed of a broad base of beta barrels that cradle the DNA, and a narrow bridge that serves to protect the double strand break from base pairing with other DNA base pairs and degradation <ref name="Walker"/>. There is little interaction between the ring and the backbone or base pairs of DNA; instead, the ring associates with DNA by the cradle fitting into the major grooves of the helix <ref name="Walker"/>. The positive electrostatic charge caused by polarization of the ring also allows the negatively charged backbone of DNA to be guided into the correct position <ref name="Walker"/> | + | The <scene name='56/567269/Ku_ring/1'>Ku Ring</scene> is composed of a broad base of beta barrels that cradle the DNA, and a narrow bridge that serves to protect the double strand break from base pairing with other DNA base pairs and degradation <ref name="Walker"/>. There is little interaction between the ring and the backbone or base pairs of DNA; instead, the ring associates with DNA by the cradle fitting into the major grooves of the helix <ref name="Walker"/>. The positive electrostatic charge caused by polarization of the ring also allows the negatively charged backbone of DNA to be guided into the correct position <ref name="Walker"/>. The Ku protein also has a high affinity to DNA due to its form being preset for the helix. As a result of the asymmetric ring, there is a strong preference (Kd value of 1.5 to 4 X 10^-10 M<ref name="Walker"/>) for the Ku ring to slide onto the ends of DNA <ref name="Walker"/>. In addition, other asymmetric features, such as a abundance of Asp residues on the N terminus of the Ku Ring, prevent the Ku protein from sliding further on the DNA helix. While wrapping over the entire helix, the Ku ring is thin over the bridge, allowing ligases and polymerases to efficiently interact in [http://en.wikipedia.org/wiki/Non-homologous_end_joining non-homologous end joining (NHEJ)]. <ref name="Walker"/> |
== Domains == | == Domains == | ||
| Line 29: | Line 29: | ||
=== α/β-Domain === | === α/β-Domain === | ||
| - | Contained inside the <scene name='56/567269/Ku70_dimer/2'>α/β-Domain</scene> is a [ | + | Contained inside the <scene name='56/567269/Ku70_dimer/2'>α/β-Domain</scene> is a [http://en.wikipedia.org/wiki/Rossman_fold Rossman fold] at the N terminus that is used to bind nucleotides in DNA.<ref name="Walker"/> |
In terms of protein structure, the α/β-Domain contributes little to the dimer interface between the subunits. | In terms of protein structure, the α/β-Domain contributes little to the dimer interface between the subunits. | ||
The C terminus of the domain can be bound to other repair molecules, using the α/β-Domain as a scaffold.<ref name="Walker"/> | The C terminus of the domain can be bound to other repair molecules, using the α/β-Domain as a scaffold.<ref name="Walker"/> | ||
| Line 50: | Line 50: | ||
The <scene name='56/567269/Ku70_dimer/6'>DNA binding ring</scene> on the open end of DNA is associated with the Ku70 subunit. | The <scene name='56/567269/Ku70_dimer/6'>DNA binding ring</scene> on the open end of DNA is associated with the Ku70 subunit. | ||
By binding DNA, Ku realigns the the strands and protects the molecule from degradation and unwanted bonds while NHEJ occurs.<ref name="Walker"/> | By binding DNA, Ku realigns the the strands and protects the molecule from degradation and unwanted bonds while NHEJ occurs.<ref name="Walker"/> | ||
| - | The regulation of the DNA binding ring of Ku is still under research, with data supporting oxidative stress and redox reactions decreasing the association of the Ku heterodimer with bound DNA through alterations in cysteine residues on the Ku70 subunit | + | The regulation of the DNA binding ring of Ku is still under research, with data supporting oxidative stress and redox reactions decreasing the association of the Ku heterodimer with bound DNA through alterations in cysteine residues on the Ku70 subunit. <ref name="source3"/> <ref name="source4"> PMID: 14585978</ref> |
Revision as of 22:07, 4 November 2013
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001 Aug 9;412(6847):607-14. PMID:11493912 doi:10.1038/35088000
- ↑ Bennett SM, Neher TM, Shatilla A, Turchi JJ. Molecular analysis of Ku redox regulation. BMC Mol Biol. 2009 Aug 28;10:86. doi: 10.1186/1471-2199-10-86. PMID:19715578 doi:http://dx.doi.org/10.1186/1471-2199-10-86
- ↑ 3.0 3.1 3.2 Polotnianka RM, Li J, Lustig AJ. The yeast Ku heterodimer is essential for protection of the telomere against nucleolytic and recombinational activities. Curr Biol. 1998 Jul 2;8(14):831-4. PMID:9663392
- ↑ 4.0 4.1 Bertuch AA, Lundblad V. The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini. Mol Cell Biol. 2003 Nov;23(22):8202-15. PMID:14585978