From Proteopedia
(Difference between revisions)
proteopedia linkproteopedia link
|
|
| Line 12: |
Line 12: |
| | ==About this Structure== | | ==About this Structure== |
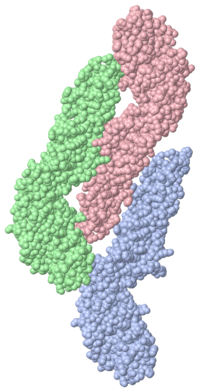
| | Dengue Virus inside Cell<StructureSection load='1ok8' size='500' side='right' caption='Dengue virus inside cell (PDB entry [[1ok8]])' scene=''>When the virus is in its infectious form the surface is smooth, but as it is exposed to the acidic environment of the cell causes the proteins to snap into a trimeric spike so as to penetrate and then fuze with the lysozome membrane. | | Dengue Virus inside Cell<StructureSection load='1ok8' size='500' side='right' caption='Dengue virus inside cell (PDB entry [[1ok8]])' scene=''>When the virus is in its infectious form the surface is smooth, but as it is exposed to the acidic environment of the cell causes the proteins to snap into a trimeric spike so as to penetrate and then fuze with the lysozome membrane. |
| - | After the lysozome membrane has been penetrated the RNA is injected and the infection begins
| + | The trimer model of the Dengue virus is extracted in many different ways. The one observed experiment was separated by detergent extraction. The model shows a chloride ion liganded by three amide nitrogens from Lys-110. The chloride is believed to dissolve away the liposome on the trimer tip. The tip of the trimer, or <scene name='56/565763/Trimer/3'>fusion loop</scene>, displays three hydrophobic residues, Trp-101, Lys-107, and Phe-108. |
| - | After a person is infected with dengue, they develop an immune response that produces specific antibodies that prevent the virus from binding to macrophage cells and gaining entry.
| + | Due to this dissolution from the chloride molecule, the three-fold –clustered membrane tip does not tightly bind together and thereby does not penetrate very deep into the host cell membrane. The fusion loop is thinking to be held into the membrane by an “aromatic anchor” formed by Trp-101 and Phe-108 |
| - | However, if another subtype of dengue virus infects the individual, the virus will activate the immune system to attack it as if it was the first subtype, producing the same antibodies as before, unfortunately these do not work on the subtype.
| + | |
| - | The immune system is tricked because the four subtypes have very similar surface antigens. The antibodies bind to the surface proteins but do not inactivate the virus. | + | |
| - | The human body is dependant on the right antibody response, and because it is not the right response the virus is not inactivated.
| + | |
| - | The immune response attracts numerous macrophages and the virus proceeds to infect them. | + | |
| - | This makes the viral infection much more acute, and the body begins to release cytokines (small cell-signaling protein molecules that are secreted by the glial cells of the nervous system) which causes the endothelial tissues (the layer of cells lining the interior surface of all blood vessels, from the heart to the smallest capillary) to become permeable, it then turns into Dengue Haemorrhagic Fever, which is deadly.
| + | |
| - | In the infectious form of the virus, the envelope protein lays flat on the surface of the virus, forming a smooth coat with icosahedral symmetry. However, when the virus is carried into the cell and into lysozomes, the acidic environment causes the protein to snap into a different shape, assembling into trimeric spike, as shown at the bottom from PDB entry 1ok8. Several hydrophobic amino acids at the tip of this spike, colored bright red here, insert into the lysozomal membrane and cause the virus membrane to fuse with lysozome. This releases the RNA into the cell and infection starts. The hemagglutinin protein on the surface of influenza virus plays a similar role, but the two proteins use entirely different mechanisms to perform a similar task.
| + | |
| | </StructureSection> | | </StructureSection> |
| | | | |
Revision as of 20:26, 11 November 2013
Dengue Virus
|
|
| 1k4r, resolution 24.00Å ()
|
| Related:
| 1svb
|
| Structural annotation:
|
| Resources:
| InterPro : Ipr009003, Ipr011492, Ipr000752, Ipr001122, Ipr001157, Ipr011998, Ipr011999, Ipr013754, Ipr014001, Ipr014021, Ipr014412, Ipr014756, Ipr002535, Ipr002877, Ipr007094, Ipr001528, Ipr001650, Ipr001850, Ipr000069, Ipr000208, Ipr000336, Ipr000404, Ipr000487
Pfam : PF02832, PF00869
SCOP : d1k4ra_, d1k4rb_, d1k4rc_
UniProt : P07720
|
|
|
|
| Resources:
| FirstGlance, OCA, RCSB, PDBsum
|
| Coordinates:
| save as pdb, mmCIF, xml
|
Structure of Dengue Virus
Publication Abstract from PubMed
The first structure of a flavivirus has been determined by using a combination of cryoelectron microscopy and fitting of the known structure of glycoprotein E into the electron density map. The virus core, within a lipid bilayer, has a less-ordered structure than the external, icosahedral scaffold of 90 glycoprotein E dimers. The three E monomers per icosahedral asymmetric unit do not have quasiequivalent symmetric environments. Difference maps indicate the location of the small membrane protein M relative to the overlaying scaffold of E dimers. The structure suggests that flaviviruses, and by analogy also alphaviruses, employ a fusion mechanism in which the distal beta barrels of domain II of the glycoprotein E are inserted into the cellular membrane.
Structure of dengue virus: implications for flavivirus organization, maturation, and fusion., Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH, Cell. 2002 Mar 8;108(5):717-25. PMID:11893341
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
Etiology
Dengue virus is a mosquito borne illness and is a major threat in most of the tropical and sub-tropical countries around the world. There are four related subtypes of the Dengue virus. Dengue is not transmitted directly from person-to-person and symptoms range from a mild fever, to incapacitating high fever, with severe headache, pain behind the eyes, muscle and joint pain, and rash. There is no vaccine or any specific medicine to treat dengue. People who have dengue fever should rest, drink plenty of fluids and reduce the fever using paracetamol or see a doctor.
About this Structure
Dengue Virus inside Cell
| When the virus is in its infectious form the surface is smooth, but as it is exposed to the acidic environment of the cell causes the proteins to snap into a trimeric spike so as to penetrate and then fuze with the lysozome membrane.
The trimer model of the Dengue virus is extracted in many different ways. The one observed experiment was separated by detergent extraction. The model shows a chloride ion liganded by three amide nitrogens from Lys-110. The chloride is believed to dissolve away the liposome on the trimer tip. The tip of the trimer, or , displays three hydrophobic residues, Trp-101, Lys-107, and Phe-108.
Due to this dissolution from the chloride molecule, the three-fold –clustered membrane tip does not tightly bind together and thereby does not penetrate very deep into the host cell membrane. The fusion loop is thinking to be held into the membrane by an “aromatic anchor” formed by Trp-101 and Phe-108
|
Dengue NS5 Protein
| The NS5 protein is a 900-residue peptide, which contains a methyltransferase domain. This protein plays an important role in the Dengue virus replication. The protein not only functions as a methyltransferase, but also as a RNA polymerase. The NS5 protein also contains guanylyltransferase activities, which, along with methyltransferase, help protect the viral genome and create efficient protein translation.
There are four serotypes of the Dengue virus, and the NS5 protein is most prominent in the Dengue-2-serotype, helping it with its pathogenesis.
Dengue-2 NS5 contains an also has an which is considered a "S-adenosyl methionine-dependent methyltransferase fold" structure. The S-adenosyl methionine ligand is methylated in the methyltransferase domain, creating S-adenosyl-l-homocysteine ()as a by-product.
In the NS5 protein, there is another binding site for GTP. This binding of GTP is done in the N-terminal domain on the protein. The is to be a cap-binding site for the Dengue-2-methyltransferase. Overall the GTP is required for translation, transcription and replication processes.
|
Dengue NS3/NS2B Protein
| The NS3 protease is a serine protease that can also function as a RNA helicase and RTPase/NTPase. The enzymatic function of this protease is important for the Dengue virus to replicate. This enzyme of the virus is also a potential target for vaccines and antiviral drugs.
The catalytic triad , is found between these two β-barrels, and its activity is dependent on the presence of the . This cofactor wraps around the NS3 protease domain and becomes part of the active site. The NS2B cofactor is critical for proteolytic activation of the NS3 protease. The NS3 protease is made up of an extensive network of hydrogen bond and hydrophobic interaction, making it very rigid. NS2B is also important in contributing to substrate binding. This implies that the NS2B cofactor acts as an enzyme activator as well as being directly involved in substrate binding/interactions.
|
Reference
- Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002 Mar 8;108(5):717-25. PMID:11893341
Categories: Dengue Virus | RCSB PDB Molecule of the Month | Viruses | Baker, T S. | Chipman, P R. | Corver, J. | Jones, C T. | Kuhn, R J. | Lenches, E. | Mukhopadhyay, S. | Pletnev, S V. | Rossmann, M G. | Strauss, E G. | Strauss, J H. | Zhang, W. | Dengue virus | Flaviviridae | Flavivirus | Glycoprotein e from tick-borne encephalitis virus | Icosahedral virus | Virus