This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Andrew Wills/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
==Active Sites and Mechanism== | ==Active Sites and Mechanism== | ||
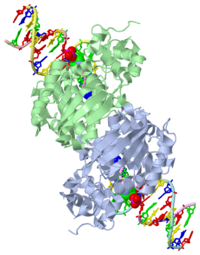
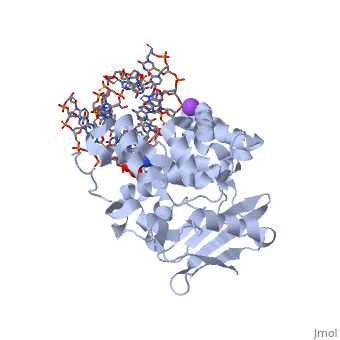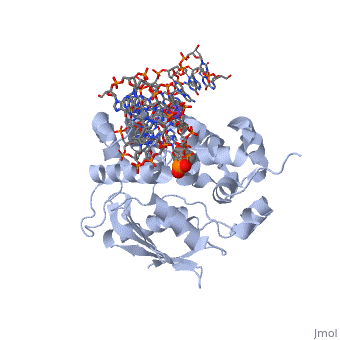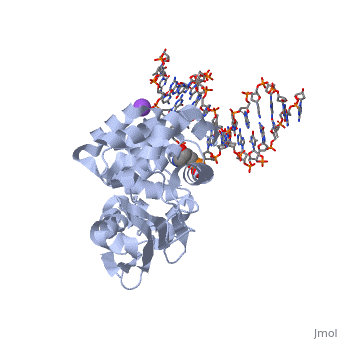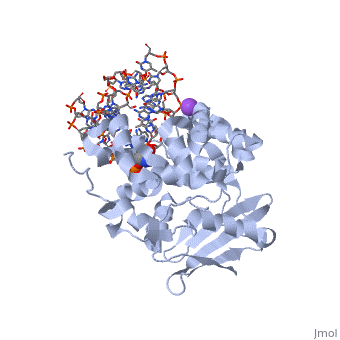
The HhH segment of AlkA connects to the phosphodiester backbone of DNA by hydrogen bonding and <scene name='56/566536/Sodium_ion_interaction/1'>sodium ion interaction</scene>. The binding of the HhH segment to DNA stabilizes the damaged base and creates a 66 degree bend away from the protein that widens the minor groove of DNA.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Leu_125/4'>Leu125</scene> fits into the minor groove between base pairs and allows the alkylated base to be flipped into the active site.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> | The HhH segment of AlkA connects to the phosphodiester backbone of DNA by hydrogen bonding and <scene name='56/566536/Sodium_ion_interaction/1'>sodium ion interaction</scene>. The binding of the HhH segment to DNA stabilizes the damaged base and creates a 66 degree bend away from the protein that widens the minor groove of DNA.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Leu_125/4'>Leu125</scene> fits into the minor groove between base pairs and allows the alkylated base to be flipped into the active site.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> | ||
| - | The AlkA active site is located where the second and third domains are separated by a deep nonpolar cleft that is lined with the aromatic side chains Phe18, Tyr273, Trp272, Tyr222, and Trp218.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> These side chains create a <scene name='56/566536/Binding_pocket/2'>DNA binding pocket</scene> for the alkylated base.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> Once the damaged base is in the active site, <scene name='56/566536/Trp_272/ | + | The AlkA active site is located where the second and third domains are separated by a deep nonpolar cleft that is lined with the aromatic side chains Phe18, Tyr273, Trp272, Tyr222, and Trp218.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> These side chains create a <scene name='56/566536/Binding_pocket/2'>DNA binding pocket</scene> for the alkylated base.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> Once the damaged base is in the active site, <scene name='56/566536/Trp_272/3'>Trp272</scene> stabilizes the flipped out alkylated base in the binding pocket by aromatic ring-stacking interactions.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Asp_238/1'>Asp238</scene> is essential for allowing the reaction to proceed, and points into the nonpolar pocket in order to allow stabilization of a carbocation intermediate. This stabilization is what allows the cleavage of the glycosidic bond on the damaged base.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> |
==DNA Interaction== | ==DNA Interaction== | ||
Revision as of 03:07, 13 November 2013
| |||||||||
| 1diz, resolution 2.50Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Non-Standard Residues: | |||||||||
| Activity: | DNA-3-methyladenine glycosylase II, with EC number 3.2.2.21 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
CRYSTAL STRUCTURE OF E. COLI 3-METHYLADENINE DNA GLYCOSYLASE (ALKA) COMPLEXED WITH DNA
| |||||||||||
See Also
References
- ↑ 1.0 1.1 Berg, Jeremy, Tymoczko John, and Lubert Stryer. Biochemistry. 6th. New York: W.H. Freeman and Company, 2007. 806-808. Print.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.
- ↑ 4.0 4.1 4.2 4.3 Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print.