User:Andrew Wills/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
==Active Sites and Mechanism== | ==Active Sites and Mechanism== | ||
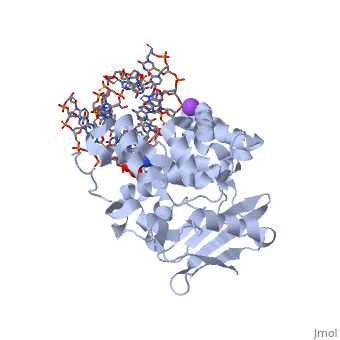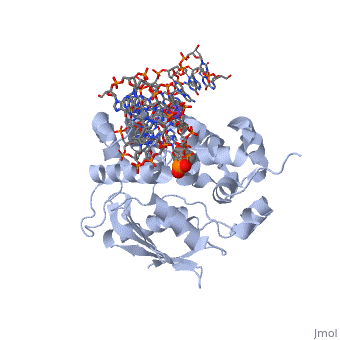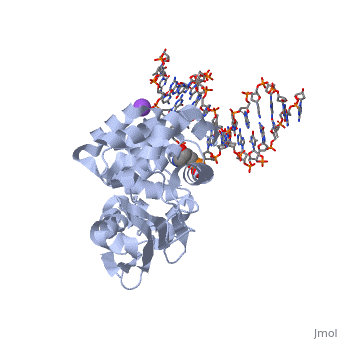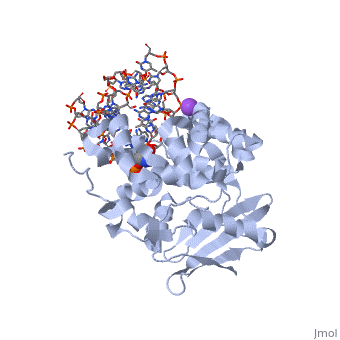
The HhH segment of AlkA connects to the phosphodiester backbone of DNA by hydrogen bonding and <scene name='56/566536/Sodium_ion_interaction/1'>sodium ion interaction</scene>. The binding of the HhH segment to DNA stabilizes the damaged base and creates a 66 degree bend away from the protein that widens the minor groove of DNA.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Leu_125/4'>Leu125</scene> fits into the minor groove between base pairs and allows the alkylated base to be flipped into the active site.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> | The HhH segment of AlkA connects to the phosphodiester backbone of DNA by hydrogen bonding and <scene name='56/566536/Sodium_ion_interaction/1'>sodium ion interaction</scene>. The binding of the HhH segment to DNA stabilizes the damaged base and creates a 66 degree bend away from the protein that widens the minor groove of DNA.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Leu_125/4'>Leu125</scene> fits into the minor groove between base pairs and allows the alkylated base to be flipped into the active site.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> | ||
| - | The AlkA active site is located where the second and third domains are separated by a deep nonpolar cleft that is lined with the aromatic side chains Phe18, Tyr273, Trp272, Tyr222, and Trp218.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> These side chains create a <scene name='56/566536/Binding_pocket/2'>DNA binding pocket</scene> for the alkylated base.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> Once the damaged base is in the active site, <scene name='56/566536/Trp_272/3'>Trp272</scene> stabilizes the flipped out alkylated base in the binding pocket by aromatic ring-stacking interactions.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Asp_238/ | + | The AlkA active site is located where the second and third domains are separated by a deep nonpolar cleft that is lined with the aromatic side chains Phe18, Tyr273, Trp272, Tyr222, and Trp218.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> These side chains create a <scene name='56/566536/Binding_pocket/2'>DNA binding pocket</scene> for the alkylated base.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> Once the damaged base is in the active site, <scene name='56/566536/Trp_272/3'>Trp272</scene> stabilizes the flipped out alkylated base in the binding pocket by aromatic ring-stacking interactions.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Asp_238/2'>Asp238</scene> is essential for allowing the reaction to proceed, and points into the nonpolar pocket in order to allow stabilization of a carbocation intermediate. This stabilization is what allows the cleavage of the glycosidic bond on the damaged base.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> |
==DNA Interaction== | ==DNA Interaction== | ||
Revision as of 03:12, 13 November 2013
| |||||||||
| 1diz, resolution 2.50Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Non-Standard Residues: | |||||||||
| Activity: | DNA-3-methyladenine glycosylase II, with EC number 3.2.2.21 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
CRYSTAL STRUCTURE OF E. COLI 3-METHYLADENINE DNA GLYCOSYLASE (ALKA) COMPLEXED WITH DNA
| |||||||||||
See Also
References
- ↑ 1.0 1.1 Berg, Jeremy, Tymoczko John, and Lubert Stryer. Biochemistry. 6th. New York: W.H. Freeman and Company, 2007. 806-808. Print.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.
- ↑ 4.0 4.1 4.2 4.3 Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print.