We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Andrew Wills/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
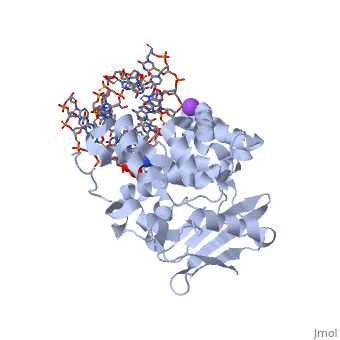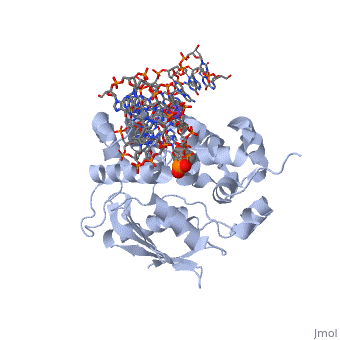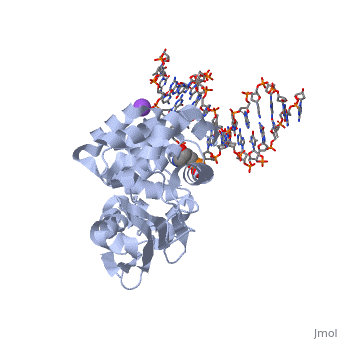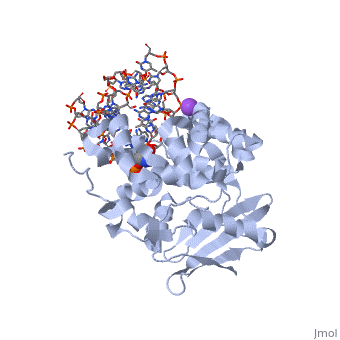
The AlkA protein stabilizes DNA by polar and nonpolar interactions with the DNA backbone. There are few positively charged residues on the AlkA’s binding surface that could interact with the DNA backbone, which is why the majority of polar interactions involve the DNA strand containing the alkylated base. | The AlkA protein stabilizes DNA by polar and nonpolar interactions with the DNA backbone. There are few positively charged residues on the AlkA’s binding surface that could interact with the DNA backbone, which is why the majority of polar interactions involve the DNA strand containing the alkylated base. | ||
| - | DNA is held in place by interactions with the HhH segment, which acts as a platform to create the 66 degree bend in DNA mentioned earlier. The bending of the DNA relieves tensional strain created by the widening of the minor groove for base flipping.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> Amide nitrogens on residues Gly214, Gly216, and Thr219 <scene name='56/566536/Hydrogen_bonding/ | + | DNA is held in place by interactions with the HhH segment, which acts as a platform to create the 66 degree bend in DNA mentioned earlier. The bending of the DNA relieves tensional strain created by the widening of the minor groove for base flipping.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> Amide nitrogens on residues Gly214, Gly216, and Thr219 <scene name='56/566536/Hydrogen_bonding/5'>hydrogen bond</scene> with DNA’s phosphodiester backbone, while residues Gln210, Phe212, and Ile215 <scene name='56/566536/Coordinate_sodium_ion/6'>coordinate</scene> the sodium metal ion that interacts with DNA further stabilize it in the molecule.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> |
In order to keep the DNA strand bound to the protein, AlkA depends on van der Waals interactions on the minor groove of DNA. The van der Waals interactions play a major role in AlkA’s preference for double stranded DNA.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> An example of this may be seen with the Pro175 wedged into the minor groove of DNA and anchoring it by van der Waals interctions.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> | In order to keep the DNA strand bound to the protein, AlkA depends on van der Waals interactions on the minor groove of DNA. The van der Waals interactions play a major role in AlkA’s preference for double stranded DNA.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> An example of this may be seen with the Pro175 wedged into the minor groove of DNA and anchoring it by van der Waals interctions.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> | ||
Revision as of 13:55, 21 November 2013
| |||||||||
| 1diz, resolution 2.50Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Non-Standard Residues: | |||||||||
| Activity: | DNA-3-methyladenine glycosylase II, with EC number 3.2.2.21 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
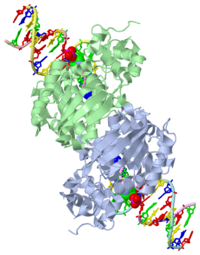
CRYSTAL STRUCTURE OF E. COLI 3-METHYLADENINE DNA GLYCOSYLASE (ALKA) COMPLEXED WITH DNA
| |||||||||||
See Also
References
- ↑ 1.0 1.1 Berg, Jeremy, Tymoczko John, and Lubert Stryer. Biochemistry. 6th. New York: W.H. Freeman and Company, 2007. 806-808. Print.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.
- ↑ 4.0 4.1 4.2 4.3 Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print.