Maureen E. Hill/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Caspase-7 Structure == | == Caspase-7 Structure == | ||
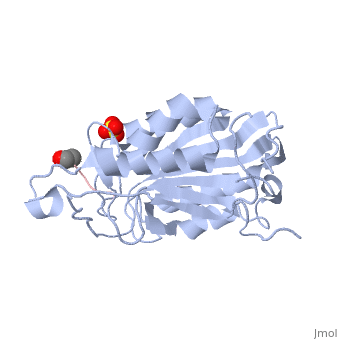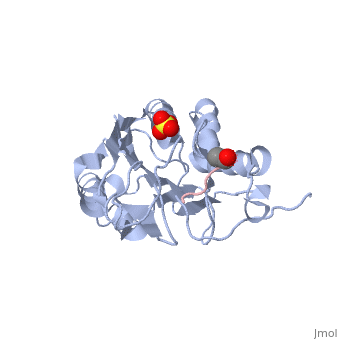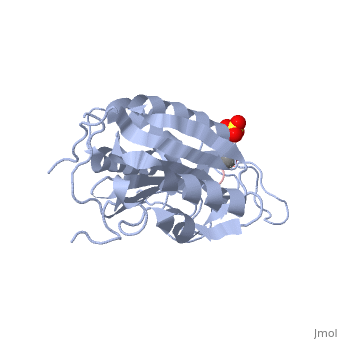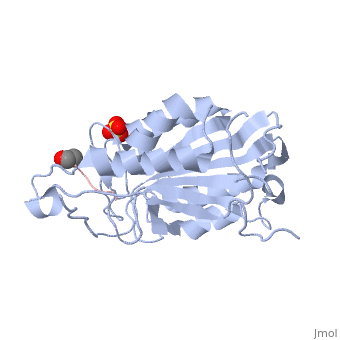
| - | Caspases are crystallized as homodimers. Each monomer contains a large (~20 kDa) and a small (~10 kDa) subunit. The <scene name='56/566502/Active_site_conformation/1'>active site</scene> is made up of four flexible loops which include L2, L3 and L4 from one half of the dimer that interact with L2' from the opposite half of the dimer. In the <scene name='56/566502/Procaspase-7_zymogen/1'>procaspase-7 zymogen</scene>, the loops are disordered, which prevents substrate binding. Upon cleavage at the intersubunit linker, the active-site loop bundle becomes partially ordered, whereas L2' stays in the inactive, down conformation. At this point, caspase-7 may bind either substrate or allosteric inhibitors. Substrate binding forces L2' to move upward, creating a foundation beneath the L2 bundle. However, it has been shown that if caspase-7 were to bind an allosteric inhibitor, such as 5-Fluoro-1H-indole-2-carboxylic acid (2-mercapto-ethyl)-amide (FICA) or 2-(2,4-Dichlorophenoxy)-N-(2-mercapto-ethyl)-acetamide (DICA), at the dimer-interface cavity, the L2' loop would be locked in the down conformation, thereby inactivating the enzyme. | + | Caspases are crystallized as homodimers. Each monomer contains a large (~20 kDa) and a small (~10 kDa) subunit. The <scene name='56/566502/Active_site_conformation/1'>active site</scene> is made up of four flexible loops which include L2, L3 and L4 from one half of the dimer that interact with L2' from the opposite half of the dimer. In the <scene name='56/566502/Procaspase-7_zymogen/1'>procaspase-7 zymogen</scene>, the loops are disordered, which prevents substrate binding. Upon cleavage at the intersubunit linker, the active-site loop bundle becomes partially ordered, whereas L2' stays in the inactive, down conformation. At this point, caspase-7 may bind either substrate or allosteric inhibitors. Substrate binding forces L2' to move upward, creating a foundation beneath the L2 bundle. However, it has been shown that if caspase-7 were to bind an allosteric inhibitor, such as 5-Fluoro-1H-indole-2-carboxylic acid (2-mercapto-ethyl)-amide ('''FICA''') or 2-(2,4-Dichlorophenoxy)-N-(2-mercapto-ethyl)-acetamide ('''DICA'''), at the dimer-interface cavity, the L2' loop would be locked in the down conformation, thereby inactivating the enzyme. |
<scene name='56/566502/Zoom_bundle/1'>Loop bundle</scene> | <scene name='56/566502/Zoom_bundle/1'>Loop bundle</scene> | ||
Revision as of 20:51, 2 December 2013
Caspase-7 Dynamics
| |||||||||||