Sandbox Reserved 772
From Proteopedia
| Line 34: | Line 34: | ||
==Structure== | ==Structure== | ||
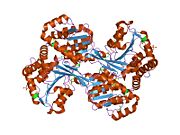
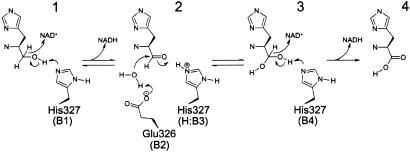
| - | HisD is a monomer, but it functions as a homodimer. The presence of Zn2+ cation is required per monomer. Each hisD monomer is made of four domains,two larger domains (globule) and two smaller domains (extending tail), whereas the intertwined dimer possibly results from domain swapping. Two domains display a very similar incomplete Rossmann fold that suggests an ancient event of gene duplication. Residues from both monomers form the active site. <ref name="rasmol">CITATION</ref> | + | HisD is a monomer, but it functions as a homodimer. The presence of Zn2+ cation is required per monomer. Each hisD monomer is made of four domains,two larger domains (globule) and two smaller domains (extending tail), whereas the intertwined dimer possibly results from domain swapping. Two domains display a very similar incomplete Rossmann fold that suggests an ancient event of gene duplication. Residues from both monomers form the active site. The active site, residue His-327, participates in acid-base catalysis <ref name="rasmol">CITATION</ref> |
| Line 46: | Line 46: | ||
This bifunctional enzyme converts L-histidinol to L-histidine through a L-histidinaldehyde intermediate. <ref name="rasmol">1</ref> | This bifunctional enzyme converts L-histidinol to L-histidine through a L-histidinaldehyde intermediate. <ref name="rasmol">1</ref> | ||
| - | ==References== <references /> | + | ==References== |
| + | <references /> | ||
Revision as of 23:23, 2 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
|
Contents |
Histidinol Dehydrogenase
Histidinol dehydrogenase (HDH) is coded by the structural gene hisD. Histidinol dehydrogenase catalyzes the last step in the histidine biosynthetic pathway. This pathway was found in bacteria, archaebacteria, fungi, and plants. The pathway involves the conversion of L-histidinol to L-histidine with a L-histidinaldehyde intermediate [1]
General Information
Gene Name: hisD
Organism: Escherichia coli (strain K12)
Molecular Function: Oxidoreductase
Length: 434 Amino Acids
Chains: A, B
Molecular Weight:
Isoelectric Point:
Km:
Vmax:
Structure
HisD is a monomer, but it functions as a homodimer. The presence of Zn2+ cation is required per monomer. Each hisD monomer is made of four domains,two larger domains (globule) and two smaller domains (extending tail), whereas the intertwined dimer possibly results from domain swapping. Two domains display a very similar incomplete Rossmann fold that suggests an ancient event of gene duplication. Residues from both monomers form the active site. The active site, residue His-327, participates in acid-base catalysis [1]
Related Structures: 1KAE and 1KAR
Enzymatic Mechanism
This bifunctional enzyme converts L-histidinol to L-histidine through a L-histidinaldehyde intermediate. [1]